MISSED THIS? Watch IWE 17. 12; Read Section 17.6. You can click on the Review link to access the section in your eText. Part A A solution containing sodium fluoride is mixed with one containing calcium nitrate to form a solution that is 0.015 M in NaF and 0.015 M in Ca(NO3)2. The following table shows Ksp values for a number of compounds. Will a precipitate form in the mixed solution? If so, identify the precipitate. Express your answer as a chemical formula. Enter noreaction if no precipitate is formed. Formula Ksp CACO3 4.96 x 10-9 ΑΣφ ? CaF2 1.46 x 10-10 Ca(OH)2 4.68 × 10–6 РЬСО 7.40х 10 14 OA chemical reaction does not occur for this question. PBF2 3.3 х 10 8 РЬ(ОН)2 1.43х 10 Submit Request Answer 20 MgCO3 6.82 × 10-6 MGF2 11 5.16 x 10 Provide Feedback Mg(OH)2 2.06 × 10-13
MISSED THIS? Watch IWE 17. 12; Read Section 17.6. You can click on the Review link to access the section in your eText. Part A A solution containing sodium fluoride is mixed with one containing calcium nitrate to form a solution that is 0.015 M in NaF and 0.015 M in Ca(NO3)2. The following table shows Ksp values for a number of compounds. Will a precipitate form in the mixed solution? If so, identify the precipitate. Express your answer as a chemical formula. Enter noreaction if no precipitate is formed. Formula Ksp CACO3 4.96 x 10-9 ΑΣφ ? CaF2 1.46 x 10-10 Ca(OH)2 4.68 × 10–6 РЬСО 7.40х 10 14 OA chemical reaction does not occur for this question. PBF2 3.3 х 10 8 РЬ(ОН)2 1.43х 10 Submit Request Answer 20 MgCO3 6.82 × 10-6 MGF2 11 5.16 x 10 Provide Feedback Mg(OH)2 2.06 × 10-13
Chemistry: Principles and Reactions
8th Edition
ISBN:9781305079373
Author:William L. Masterton, Cecile N. Hurley
Publisher:William L. Masterton, Cecile N. Hurley
Chapter15: Complex Ion And Precipitation Equilibria
Section: Chapter Questions
Problem 30QAP: Silver(I) sulfate (Ksp=1.2105) is used in the electroplating of silver. A 1.0-L solution is prepared...
Related questions
Question
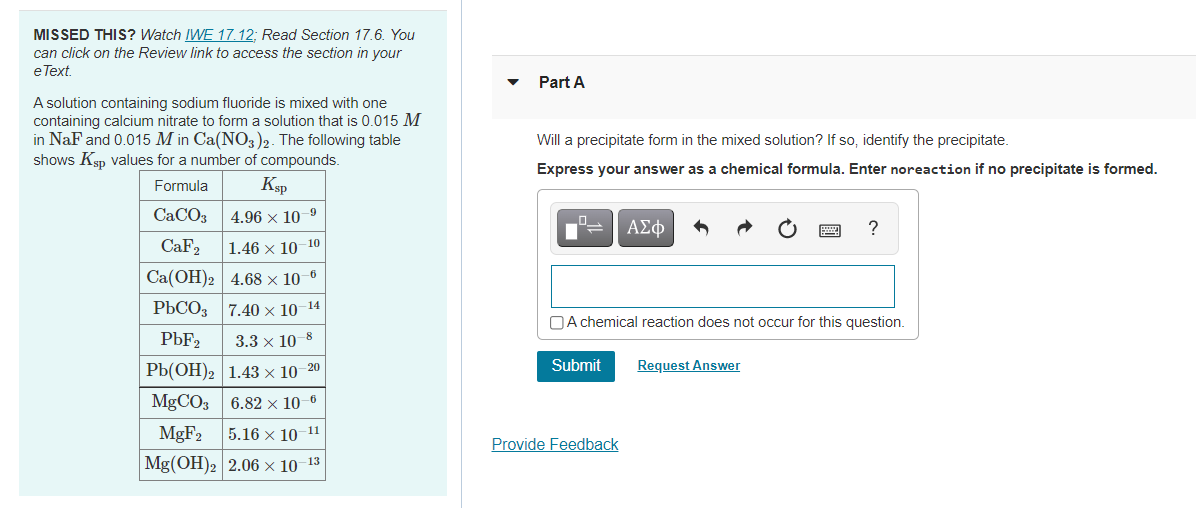
Transcribed Image Text:MISSED THIS? Watch IWE 17. 12; Read Section 17.6. You
can click on the Review link to access the section in your
eText.
Part A
A solution containing sodium fluoride is mixed with one
containing calcium nitrate to form a solution that is 0.015 M
in NaF and 0.015 M in Ca(NO3)2. The following table
shows Ksp values for a number of compounds.
Will a precipitate form in the mixed solution? If so, identify the precipitate.
Express your answer as a chemical formula. Enter noreaction if no precipitate is formed.
Formula
Ksp
CACO3
4.96 x 10-9
ΑΣφ
?
CaF2
1.46 x 10-10
Ca(OH)2 4.68 × 10–6
РЬСО 7.40х 10 14
OA chemical reaction does not occur for this question.
PBF2
3.3 х 10 8
РЬ(ОН)2 1.43х 10
Submit
Request Answer
20
MgCO3 6.82 × 10-6
MGF2
11
5.16 x 10
Provide Feedback
Mg(OH)2 2.06 × 10-13
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning