Modify the structures (if necessary) to show the predominant form of the amino acid tyrosine at each pH. Be sure to include the proper number of hydrogen atoms on heteroatoms and the correct formal charges on atoms. At pH 4.6 At pH 11.1 он H,N - CH H,N - CH CH, CH, HC CH HC CH || HC CH HC CH OH он
Modify the structures (if necessary) to show the predominant form of the amino acid tyrosine at each pH. Be sure to include the proper number of hydrogen atoms on heteroatoms and the correct formal charges on atoms. At pH 4.6 At pH 11.1 он H,N - CH H,N - CH CH, CH, HC CH HC CH || HC CH HC CH OH он
Introduction to General, Organic and Biochemistry
11th Edition
ISBN:9781285869759
Author:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Chapter22: Proteins
Section: Chapter Questions
Problem 22.49P: 22-49 Based on your knowledge of the chemical properties of amino acid side chains, suggest a...
Related questions
Question
100%
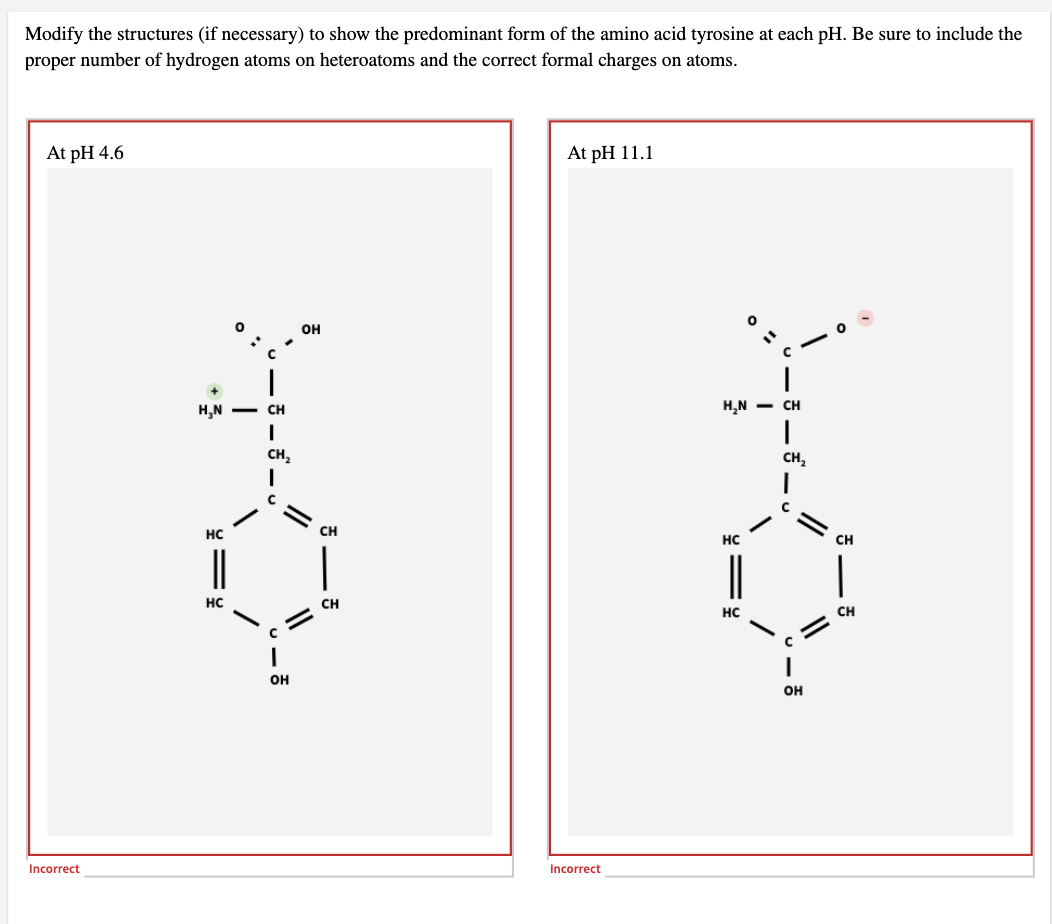
Transcribed Image Text:Modify the structures (if necessary) to show the predominant form of the amino acid tyrosine at each pH. Be sure to include the
proper number of hydrogen atoms on heteroatoms and the correct formal charges on atoms.
At pH 4.6
At pH 11.1
он
H,N - CH
H,N - CH
CH,
CH,
HC
CH
HC
CH
|
|
|
HC
CH
HC
CH
он
он
Incorrect
Incorrect
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Recommended textbooks for you

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

World of Chemistry
Chemistry
ISBN:
9780618562763
Author:
Steven S. Zumdahl
Publisher:
Houghton Mifflin College Div

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning