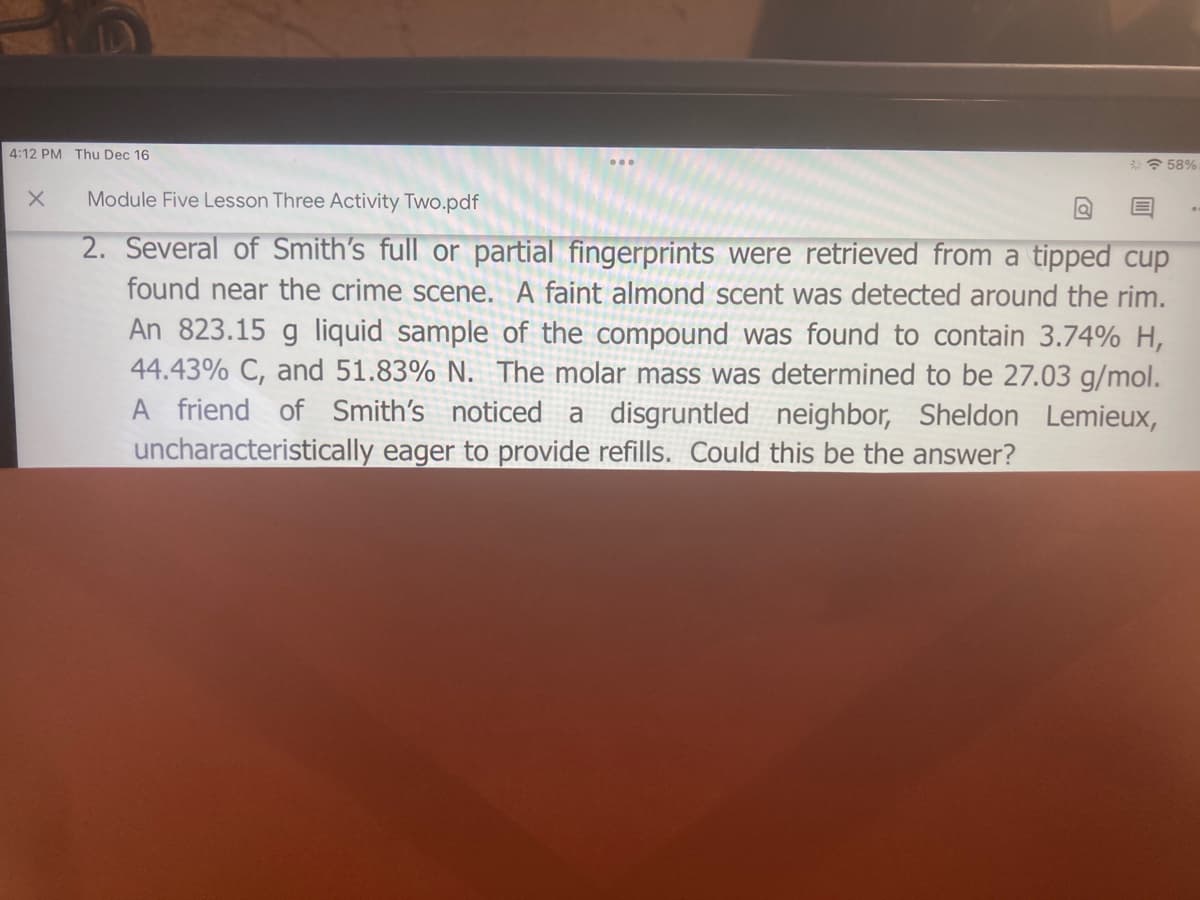
Module Five Lesson Three Activity Two.pdf 2. Several of Smith's full or partial fingerprints were retrieved from a tipped cup found near the crime scene. A faint almond scent was detected around the rim. An 823.15 g liquid sample of the compound was found to contain 3.74% H, 44.43% C, and 51.83% N. The molar mass was determined to be 27.03 g/mol. A friend of Smith's noticed a disgruntled neighbor, Sheldon Lemieux, uncharacteristically eager to provide refills. Could this be the answer?
Module Five Lesson Three Activity Two.pdf 2. Several of Smith's full or partial fingerprints were retrieved from a tipped cup found near the crime scene. A faint almond scent was detected around the rim. An 823.15 g liquid sample of the compound was found to contain 3.74% H, 44.43% C, and 51.83% N. The molar mass was determined to be 27.03 g/mol. A friend of Smith's noticed a disgruntled neighbor, Sheldon Lemieux, uncharacteristically eager to provide refills. Could this be the answer?
General Chemistry - Standalone book (MindTap Course List)
11th Edition
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Chapter5: The Gaseous State
Section: Chapter Questions
Problem 5.111QP: A radioactive metal atom decays (goes to another kind of atom) by emitting an alpha particle (He2+...
Related questions
Question
100%
Using the information from number 2, calculate the empirical and molecular formula for the scenario and then the chemical compound.
Transcribed Image Text:4:12 PM Thu Dec 16
** 58%
Module Five Lesson Three Activity Two.pdf
2. Several of Smith's full or partial fingerprints were retrieved from a tipped cup
found near the crime scene. A faint almond scent was detected around the rim.
An 823.15 g liquid sample of the compound was found to contain 3.74% H,
44.43% C, and 51.83% N. The molar mass was determined to be 27.03 g/mol.
A friend of Smith's noticed a disgruntled neighbor, Sheldon Lemieux,
uncharacteristically eager to provide refills. Could this be the answer?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning