mol AB-016 A student was given a sample of hydrated Na2CrO4 (molar mass of anhydrous Na2CrO4. 162.0 g/mol). She determined the weight of the hydrated sample to be 2.58 heating the hydrate to drive off all the water of hydration, she determined the weight of the anhydrous salt to be 1.77 g. Calculate the formula of the hydrated salt (round to the nearest whole number of water molecules/mol). After g.
mol AB-016 A student was given a sample of hydrated Na2CrO4 (molar mass of anhydrous Na2CrO4. 162.0 g/mol). She determined the weight of the hydrated sample to be 2.58 heating the hydrate to drive off all the water of hydration, she determined the weight of the anhydrous salt to be 1.77 g. Calculate the formula of the hydrated salt (round to the nearest whole number of water molecules/mol). After g.
Chapter3: Mechanisms
Section: Chapter Questions
Problem 111EQ
Related questions
Question
100%
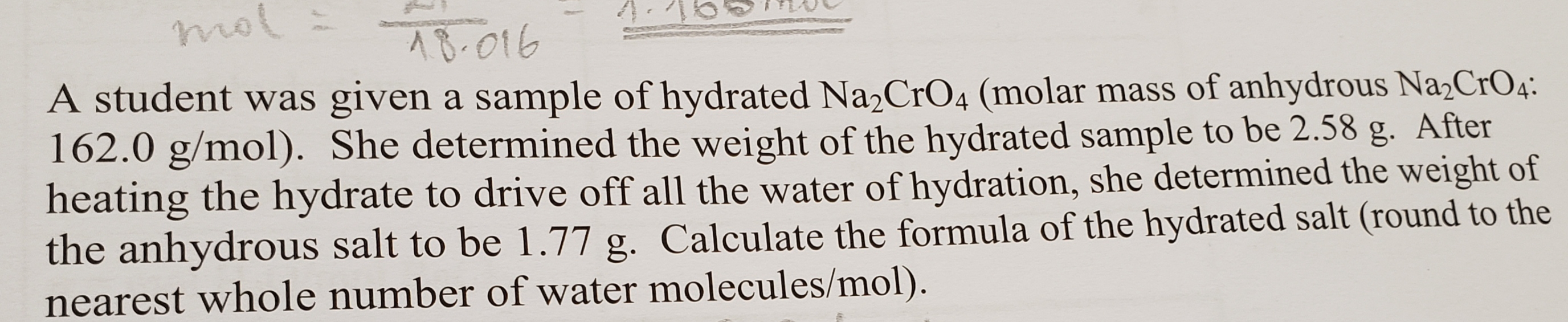
Transcribed Image Text:mol
AB-016
A student was given a sample of hydrated Na2CrO4 (molar mass of anhydrous Na2CrO4.
162.0 g/mol). She determined the weight of the hydrated sample to be 2.58
heating the hydrate to drive off all the water of hydration, she determined the weight of
the anhydrous salt to be 1.77 g. Calculate the formula of the hydrated salt (round to the
nearest whole number of water molecules/mol).
After
g.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you

