Molar masses for all compounds in the equation: K: 39.10 g/mol H₂O: 18.02 g/mol KOH:56.11 g/mol H₂: 2.02 g/mol 2K (s) + 2 H₂O (1) 2 KOH (s) + H₂ (8) You need to plan a reaction to produce 20 L of H2. What mass of potassium (K) will you use to accomplish this?
Molar masses for all compounds in the equation: K: 39.10 g/mol H₂O: 18.02 g/mol KOH:56.11 g/mol H₂: 2.02 g/mol 2K (s) + 2 H₂O (1) 2 KOH (s) + H₂ (8) You need to plan a reaction to produce 20 L of H2. What mass of potassium (K) will you use to accomplish this?
World of Chemistry, 3rd edition
3rd Edition
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Chapter9: Chemical Quantities
Section: Chapter Questions
Problem 2STP
Related questions
Question
100%
Solve like the example attached. Apply the significant figure rule to the final answer.
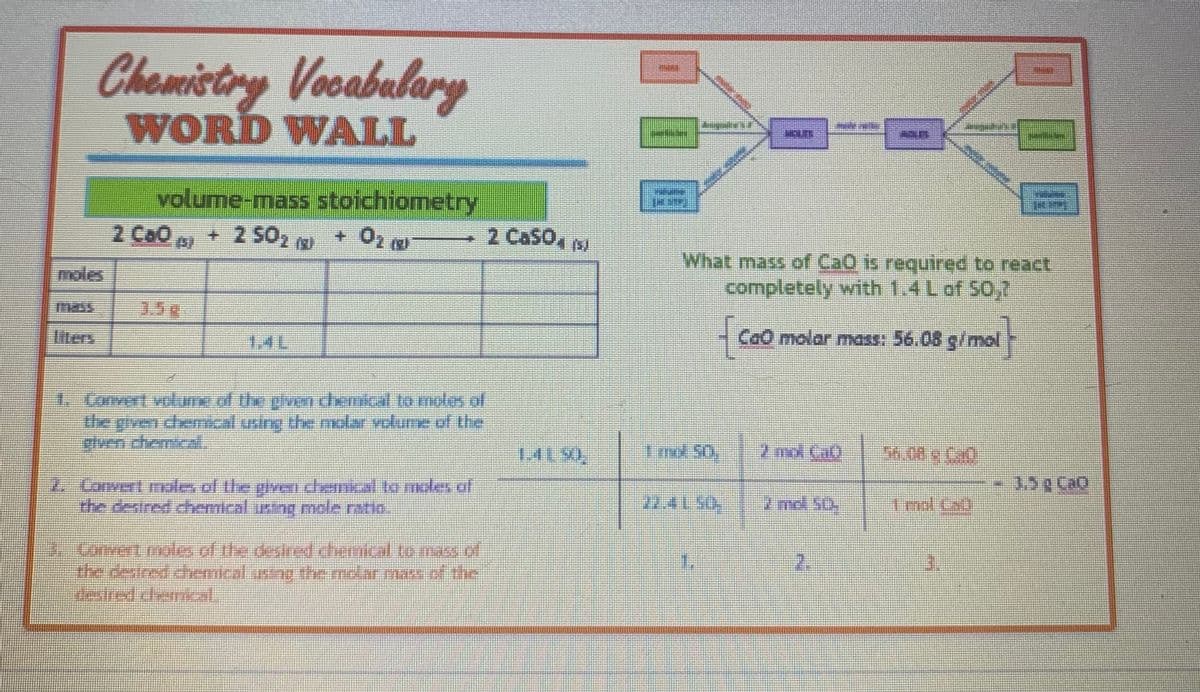
Transcribed Image Text:Chemistry Vocabulary
WORD WALL
volume-mass stoichiometry
+ 2 50₂ MU
+ O₂ (g)
1. Convent volume of the given chemical to moles of
the given chemical using the molar volume of the
2. Convert moles of the given chemical to moles of
the desired chemical using mole ratio.
3 Convert moles of the desired chemical to mass of
the desired chemical using the molar mass of the
2 Caso, (5)
1.4190
6
BRUNE
1m 50.
F
F
What mass of CaO is required to react
completely with 1.4 L of 50,2
Cao molar mass: 56.08 g/mol
7 md. GO
2 md. 50.
2
IDENT
56.08 € Cal
T
3.5g CaQ
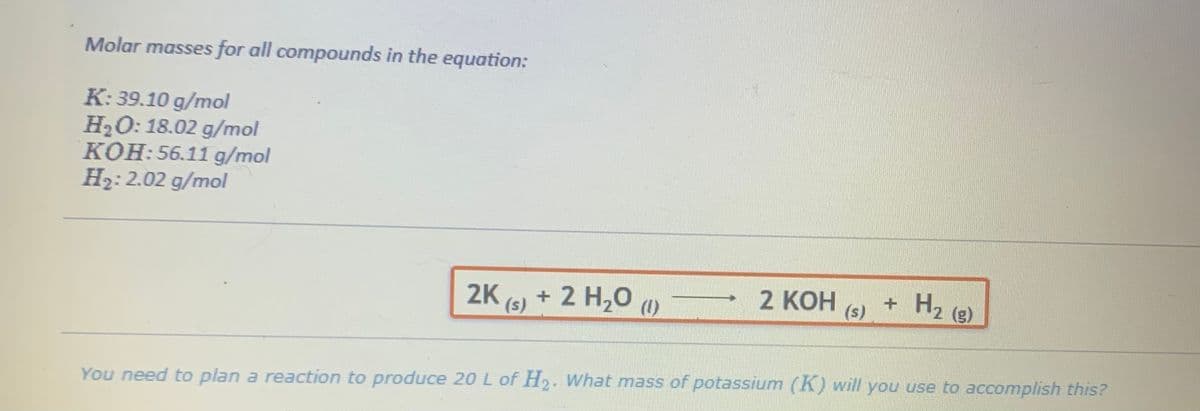
Transcribed Image Text:Molar masses for all compounds in the equation:
K: 39.10 g/mol
H₂O: 18.02 g/mol
KOH: 56.11 g/mol
H₂: 2.02 g/mol
2K (s) + 2 H₂O (1)
2 KOH
(s)
+ H₂ (g)
You need to plan a reaction to produce 20 L of H₂. What mass of potassium (K) will you use to accomplish this?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning