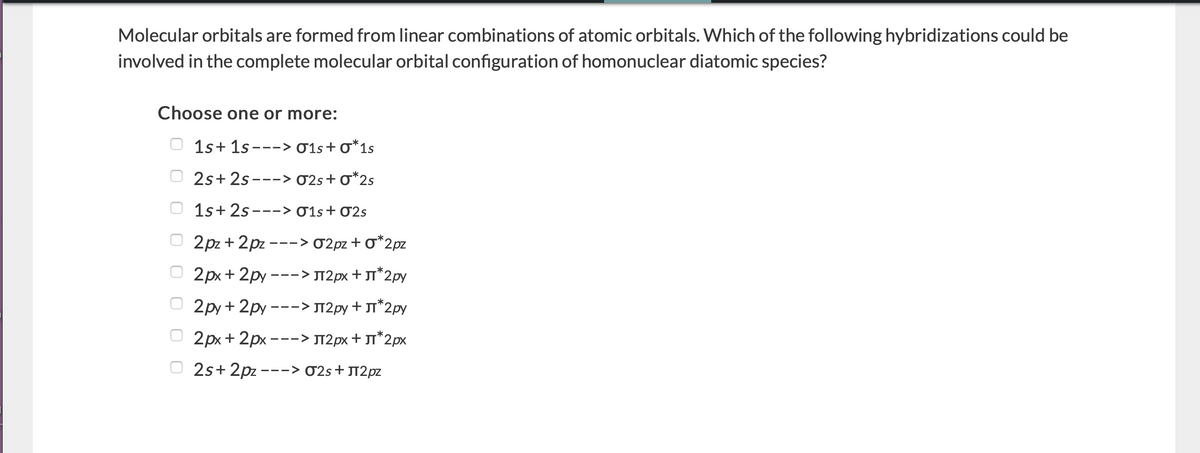
Molecular orbitals are formed from linear combinations of atomic orbitals. Which of the following hybridizations could be involved in the complete molecular orbital configuration of homonuclear diatomic species? Choose one or more: O 1s+ 1s---> 01s+o*1s O 2s+ 2s---> 02s+o*2s O 1s+ 2s---> 01s+ 02s O 2pz + 2pz ---> 02pz + o*2pz O 2px + 2py ---> T2px + JT*2py O 2py + 2py ---> T2py + T*2py O 2px + 2px ---> T2px+ T*2px O 2s+ 2pz ---> 02s+J12pz
Molecular orbitals are formed from linear combinations of atomic orbitals. Which of the following hybridizations could be involved in the complete molecular orbital configuration of homonuclear diatomic species?
Given : Different type of atomic orbitals.
To find : Best combination of hybridization for the formation of Homonuclear diatomic molecules.
Solution : As we know that, Homonuclear diatomic molecules are those molecules in which both of atoms are same. If the atoms present in the molecules are different then it is known as Hetronuclear diatomic molecules.
Example :
A + A = AA ( Homonuclear diatomic molecule)
A + B = AB (Hetronuclear diatomic molecule)
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images









