60.What is/are the purpose/s of heating sodium carbonate in 270°C for one hour before using it in the standardization of hydrochloric acid? 1. To remove carbon dioxide that is generated during the neutralization reaction II. To remove moisture III. To convert sodium barbonate to sodium bicarbonate a. I, II and III c. I only b. II only d. II and III 61. The process by which the exact concentration of a solution is determined: a. Dissolution c. Standardization d. Titration b. Neutralization 62. The function of Quality Control include the following, except: a. Planning C. Analytical control d. Inspection control b. Auditing
60.What is/are the purpose/s of heating sodium carbonate in 270°C for one hour before using it in the standardization of hydrochloric acid? 1. To remove carbon dioxide that is generated during the neutralization reaction II. To remove moisture III. To convert sodium barbonate to sodium bicarbonate a. I, II and III c. I only b. II only d. II and III 61. The process by which the exact concentration of a solution is determined: a. Dissolution c. Standardization d. Titration b. Neutralization 62. The function of Quality Control include the following, except: a. Planning C. Analytical control d. Inspection control b. Auditing
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter4: Energy And Chemical Reactions
Section: Chapter Questions
Problem 84QRT: Oxygen is not normally found in positive oxidation states, but when it is combined with fluorine in...
Related questions
Question
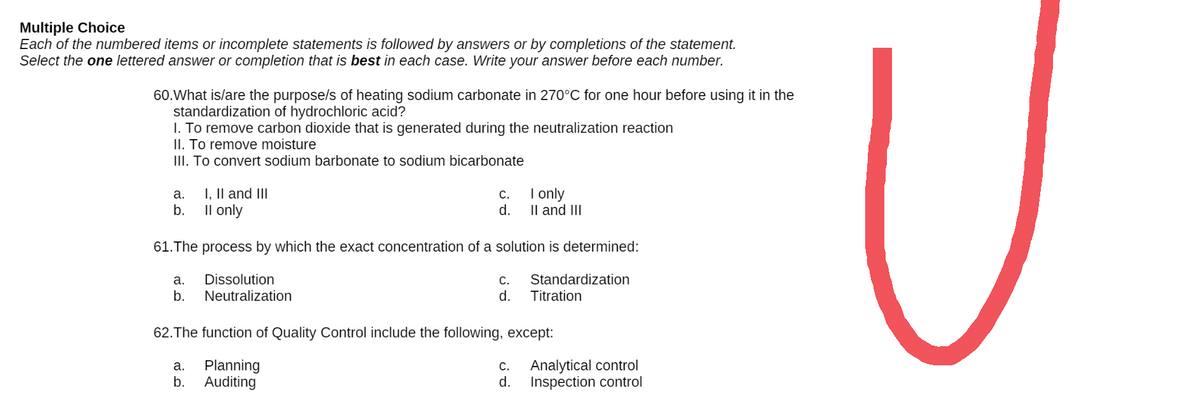
Transcribed Image Text:Multiple Choice
Each of the numbered items or incomplete statements is followed by answers or by completions of the statement.
Select the one lettered answer or completion that is best in each case. Write your answer before each number.
60. What is/are the purpose/s of heating sodium carbonate in 270°C for one hour before using it in the
standardization of hydrochloric acid?
1. To remove carbon dioxide that is generated during the neutralization reaction
II. To remove moisture
III. To convert sodium barbonate to sodium bicarbonate
a. I, II and III
C.
I only
b.
II only
d.
II and III
61. The process by which the exact concentration of a solution is determined:
a.
Dissolution
C.
Standardization
Titration
b.
Neutralization
d.
62. The function of Quality Control include the following, except:
a.
C.
Analytical control
Planning
b. Auditing
d.
Inspection control
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole


Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning