Synthesis of Dilantin by Muli-Step Synthesis Part 4- Ureabenzil Condensation Table of Reagents Molecular weight (g/mol) Mass (g) Volume (mL) density (g/mL) Amount Reagent (mmol) 210.23 1.0 4.75 benzil Sodium hydroxide (30% wiv) 34.997 1.2 loo 7.14 urea 0.432 Limiting Reagent: Identify the limiting reagent (warning, some reagents may be catalysts, and can be used in sub- stoichiometric amounts, and not all reactions have 1:1 stoichiometric coefficients) Yield of Product Calculate the % yield of product you obtained. Watch significant figures and SHOW YOUR WORKING. Overall yield of product based on benzaldehyde: Calculate the % yield of the product you obtained based on the starting material for this synthesis, benzaldehyde. Watch significant figures and SHOW YOUR WORKING.
Synthesis of Dilantin by Muli-Step Synthesis Part 4- Ureabenzil Condensation Table of Reagents Molecular weight (g/mol) Mass (g) Volume (mL) density (g/mL) Amount Reagent (mmol) 210.23 1.0 4.75 benzil Sodium hydroxide (30% wiv) 34.997 1.2 loo 7.14 urea 0.432 Limiting Reagent: Identify the limiting reagent (warning, some reagents may be catalysts, and can be used in sub- stoichiometric amounts, and not all reactions have 1:1 stoichiometric coefficients) Yield of Product Calculate the % yield of product you obtained. Watch significant figures and SHOW YOUR WORKING. Overall yield of product based on benzaldehyde: Calculate the % yield of the product you obtained based on the starting material for this synthesis, benzaldehyde. Watch significant figures and SHOW YOUR WORKING.
Chapter21: Carboxylic Acid Derivatives: Nucleophilic Acyl Substitution Reactions
Section21.SE: Something Extra
Problem 35MP
Related questions
Question
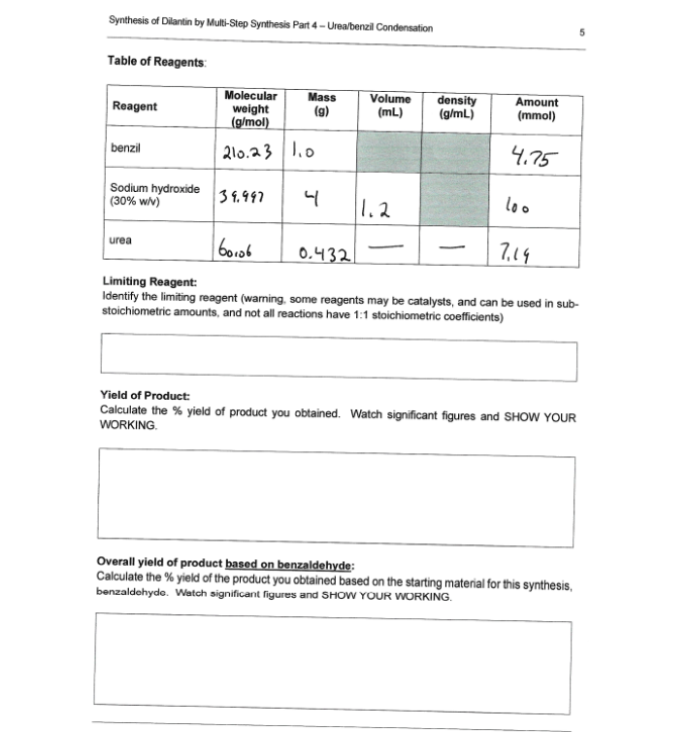
Transcribed Image Text:Synthesis of Dilantin by Multi-Step Synthesis Part 4 - Ureabenzil Condensation
Table of Reagents
Molecular
weight
(g/mol)
Mass
(g)
Volume
(mL)
density
(g/mL)
Amount
(mmol)
Reagent
benzil
210.23 1.0
4.75
Sodium hydroxide
(30% wv)
39.997
1.2
loo
urea
0.432
7,19
-
Limiting Reagent:
Identify the limiting reagent (warning, some reagents may be catalysts, and can be used in sub-
stoichiometric amounts, and not all reactions have 1:1 stoichiometric coefficients)
Yield of Product:
Calculate the % yield of product you obtained. Watch significant figures and SHOW YOUR
WORKING.
Overall yield of product based on benzaldehyde:
Calculate the % yield of the product you obtained based on the starting material for this synthesis,
benzaldehyde. Watch significant figures and SHOW YOUR WORKING.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 5 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning