NAOH (ag) + HCI NaCl (ag) + H20 (1) (aq) -> 31.88 mL of 0.09951 M NaOH was used to titrate 7.00 mL of HCI to the endpoint. Calculate the mol of HCI that reacted and the molarity of the HCI solution. SHOW WORK. Write out the problem on paper showing all conversion factors, unit cancellations, calculations, s.f., etc. Answer the questions related to the setup and calculation for this problem. Be sure to use our periodic table to calculate any molar masses needed (rounded to proper number of decimal places), otherwise your values might be slightly off and answers may be marked as incorrect. Abbreviate units as follows: grams = g, moles = mol, molarity = M Enter where each reactant is placed at the beginning of the experiment (options are buret or flask): NaOH: HCl: Use the three blanks to enter the number, unit, and substance (in this order) that appears in the numerator of the stoichiometry conversion factor. Calculate the mol of HCl that reacted and enter the value only (decimal notation, including proper s.f.). mol Calculate the molarity of the original HCl solution and enter the value only (decimal notation, including proper s.f.). M
NAOH (ag) + HCI NaCl (ag) + H20 (1) (aq) -> 31.88 mL of 0.09951 M NaOH was used to titrate 7.00 mL of HCI to the endpoint. Calculate the mol of HCI that reacted and the molarity of the HCI solution. SHOW WORK. Write out the problem on paper showing all conversion factors, unit cancellations, calculations, s.f., etc. Answer the questions related to the setup and calculation for this problem. Be sure to use our periodic table to calculate any molar masses needed (rounded to proper number of decimal places), otherwise your values might be slightly off and answers may be marked as incorrect. Abbreviate units as follows: grams = g, moles = mol, molarity = M Enter where each reactant is placed at the beginning of the experiment (options are buret or flask): NaOH: HCl: Use the three blanks to enter the number, unit, and substance (in this order) that appears in the numerator of the stoichiometry conversion factor. Calculate the mol of HCl that reacted and enter the value only (decimal notation, including proper s.f.). mol Calculate the molarity of the original HCl solution and enter the value only (decimal notation, including proper s.f.). M
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter17: Electrochemistry
Section: Chapter Questions
Problem 80AP
Related questions
Question
100%
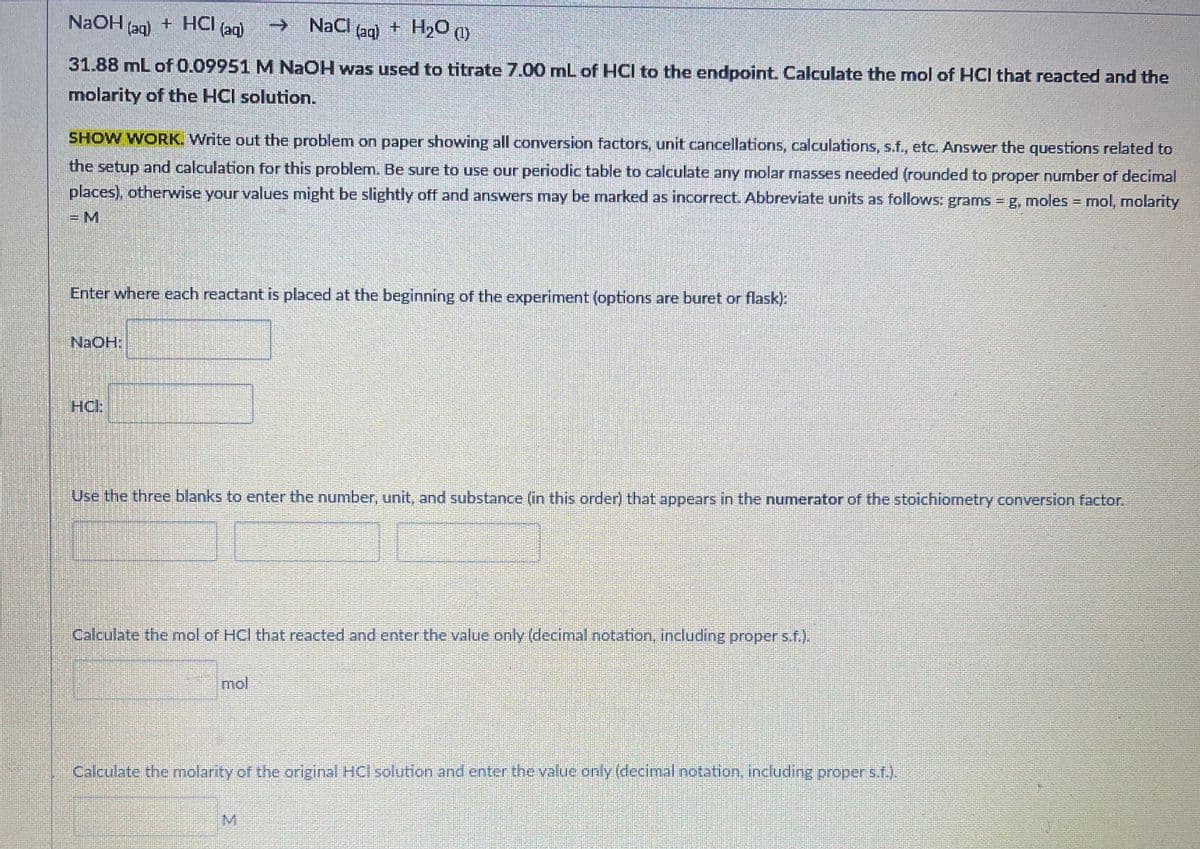
Transcribed Image Text:NAOH
(aq)
+ HCI
(aq)
->
NaCl
(aq) + H20 )
31.88 mL of 0.09951 M NAOH was used to titrate 7.00 mL of HCI to the endpoint. Calculate the mol of HCI that reacted and the
molarity of the HCl solution.
SHOW WORK. Write out the problem on paper showing all conversion factors, unit cancellations, calculations, s.f., etc. Answer the questions related to
the setup and calculation for this problem. Be sure to use our periodic table to calculate any molar masses needed (rounded to proper number of decimal
places), otherwise your values might be slightly off and answers may be marked as incorrect. Abbreviate units as follows: grams = g, moles - mol, molarity
=D
Enter where each reactant is placed at the beginning of the experiment (options are buret or flask):
HCH
Use the three blanks to enter the number, unit, and substance (in this order) that appears in the numerator of the stoichiometry conversion factor.
Calculate the mol of HCl that reacted and enter the value only (decimal notation, including proper s.f.).
mol
Calculate the molarity of the original HCl solution and enter the value only (decimal notation, including proper s.f.).
M.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning