NaOH (aq) + HCI (aq) NACI (aq) + H2O (1) Volume of 1.00M HCI (mL) 45.0 Volume of 1.00 M NAOH (mL) 50.0 Initial Temperature ("C) 22.1 Final Temperature ("C) 31.6 la) Calculate the moles of HCI used in the reaction. Moles of HCI = mol (b) Calculate the moles of NaOH used in the reaction. Moles NaOH= mol (c) Which reactant is the Limiting Reactant, HCl or NaOH? Limiting Reactant = (d) Is this acid-base neutralization reaction exothermic or endothermic? NOTE: DO Include the correct number of significant figures or you will be marked
NaOH (aq) + HCI (aq) NACI (aq) + H2O (1) Volume of 1.00M HCI (mL) 45.0 Volume of 1.00 M NAOH (mL) 50.0 Initial Temperature ("C) 22.1 Final Temperature ("C) 31.6 la) Calculate the moles of HCI used in the reaction. Moles of HCI = mol (b) Calculate the moles of NaOH used in the reaction. Moles NaOH= mol (c) Which reactant is the Limiting Reactant, HCl or NaOH? Limiting Reactant = (d) Is this acid-base neutralization reaction exothermic or endothermic? NOTE: DO Include the correct number of significant figures or you will be marked
Materials Science And Engineering Properties
1st Edition
ISBN:9781111988609
Author:Charles Gilmore
Publisher:Charles Gilmore
Chapter4: Temperature Effects On Atom Arrangements And Atom Motion
Section: Chapter Questions
Problem 4.25P
Related questions
Question
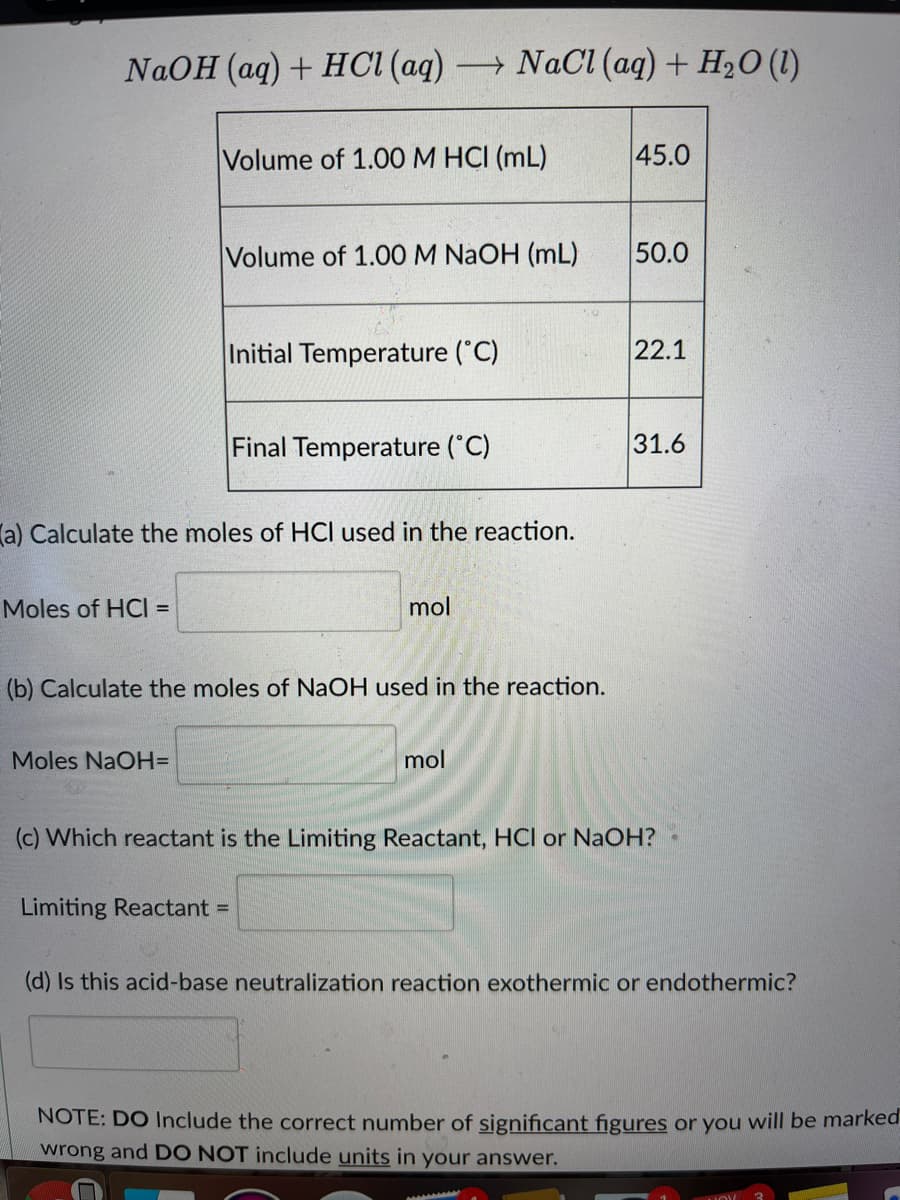
Transcribed Image Text:NaOH (aq) + HCI (aq) NACI (aq) + H2O (1)
Volume of 1.00M HCI (mL)
45.0
Volume of 1.00 M NAOH (mL)
50.0
Initial Temperature ("C)
22.1
Final Temperature (C)
31.6
la) Calculate the moles of HCI used in the reaction.
Moles of HCI =
mol
(b) Calculate the moles of NaOH used in the reaction.
Moles NaOH=
mol
(c) Which reactant is the Limiting Reactant, HCl or NaOH?
Limiting Reactant =
(d) Is this acid-base neutralization reaction exothermic or endothermic?
NOTE: DO Include the correct number of significant figures or you will be marked
wrong and DO NOT include units in your answer.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, civil-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Materials Science And Engineering Properties
Civil Engineering
ISBN:
9781111988609
Author:
Charles Gilmore
Publisher:
Cengage Learning


Solid Waste Engineering
Civil Engineering
ISBN:
9781305635203
Author:
Worrell, William A.
Publisher:
Cengage Learning,

Materials Science And Engineering Properties
Civil Engineering
ISBN:
9781111988609
Author:
Charles Gilmore
Publisher:
Cengage Learning


Solid Waste Engineering
Civil Engineering
ISBN:
9781305635203
Author:
Worrell, William A.
Publisher:
Cengage Learning,