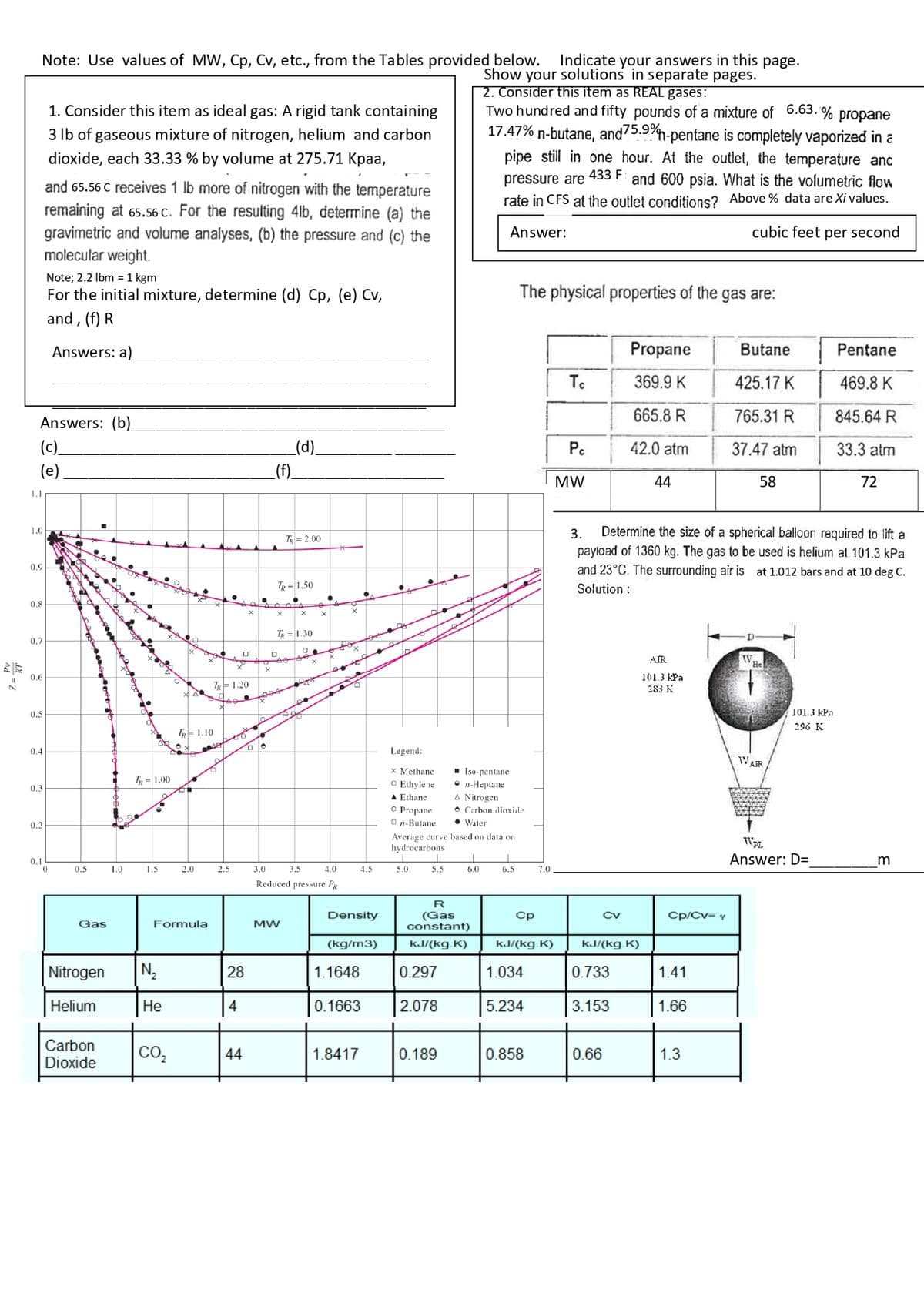
Note: Use values of MW, Cp, Cv, etc., from the Tables provided below. Indicate your answers in this page. Show your solutions in separate pages. 2. Consider this item as REAL gases: Two hundred and fifty pounds of a mixture of 6.63.% 17.47% n-butane, and 75.9%- -pentane is completely vapori 1. Consider this item as ideal gas: A rigid tank containing 3 lb of gaseous mixture of nitrogen, helium and carbon dioxide, each 33.33 % by volume at 275.71 Kpaa, and 65.56 receives 1 lh more of nitrogen with the temporoti pipe still in one hour. At the outlet, the temperat pressure are 433 F and 600 psia. What is the volume
Note: Use values of MW, Cp, Cv, etc., from the Tables provided below. Indicate your answers in this page. Show your solutions in separate pages. 2. Consider this item as REAL gases: Two hundred and fifty pounds of a mixture of 6.63.% 17.47% n-butane, and 75.9%- -pentane is completely vapori 1. Consider this item as ideal gas: A rigid tank containing 3 lb of gaseous mixture of nitrogen, helium and carbon dioxide, each 33.33 % by volume at 275.71 Kpaa, and 65.56 receives 1 lh more of nitrogen with the temporoti pipe still in one hour. At the outlet, the temperat pressure are 433 F and 600 psia. What is the volume
Introduction to Chemical Engineering Thermodynamics
8th Edition
ISBN:9781259696527
Author:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Chapter1: Introduction
Section: Chapter Questions
Problem 1.1P
Related questions
Question
UPVOTE WILL BE GIVEN. PLEASE WRITE THE COMPLETE SOLUTIONS LEGIBLY. SHOW THE STEP-BY-STEP PROCESS WITH SHORT COMMENT/EXPLANATION. ANSWER IN 3 DECIMAL PLACES.
answer #1 only.
Transcribed Image Text:2² = Z
Note: Use values of MW, Cp, Cv, etc., from the Tables provided below. Indicate your answers in this page.
Show your solutions in separate pages.
2. Consider this item as REAL gases:
Two hundred and fifty pounds of a mixture of 6.63.% propane
17.47% n-butane, and 75.9%-pentane is completely vaporized in a
pipe still in one hour. At the outlet, the temperature anc
pressure are 433 F: and 600 psia. What is the volumetric flow
rate in CFS at the outlet conditions? Above % data are Xi values.
Answer:
cubic feet per second
1.1
Answers: (b)
(c).
(e)
1.0
0.9
08
0.7
0.6
0.5
0.4
0.3
1. Consider this item as ideal gas: A rigid tank containing
3 lb of gaseous mixture of nitrogen, helium and carbon
dioxide, each 33.33 % by volume at 275.71 Kpaa,
and 65.56 C receives 1 lb more of nitrogen with the temperature
remaining at 65.56 c. For the resulting 4lb, determine (a) the
gravimetric and volume analyses, (b) the pressure and (c) the
molecular weight.
0.2
Note; 2.2 lbm = 1 kgm
For the initial mixture, determine (d) Cp, (e) Cv,
and, (f) R
Answers: a)
0.1
0
0.5
Gas
Nitrogen
Helium
Carbon
Dioxide
1.0
T=1.00
1.5
N₂
Formula
He
TR=1.10
CO₂
2.0
2.5
ADA
TR=1.20
0
4
28
44
x
(f)
3.0
(d)
TR = 2.00
T = 1.50
To = 1.30
MW
BAK
4.0
Reduced pressure PR
X
3.5
4.5
Density
(kg/m3)
1.1648
0.1663
1.8417
Legend:
X Methane
o Ethylene
■ Iso-pentane
• n-Heptane
A Nitrogen
Carbon dioxide
• Water
Average curve based on data on
hydrocarbons
▲ Ethane
o Propane
□n-Butane
5.0
5.5
R
(Gas
constant)
kJ/(kg.K)
0.297
2.078
I
6.0
0.189
The physical properties of the gas are:
6.5
Cp
kJ/(kg.K)
1.034
5.234
7.0
0.858
Tc
Pc
MW
Cv
kJ/(kg.K)
0.733
Propane
369.9 K
665.8 R
42.0 atm
3.153
0.66
44
3.
Determine the size of a spherical balloon required to lift a
payload of 1360 kg. The gas to be used is helium at 101.3 kPa
and 23°C. The surrounding air is at 1.012 bars and at 10 deg C.
Solution :
AIR
101.3 kPa
283 K
Cp/Cv=Y
1.41
1.66
Butane
425.17 K
765.31 R
37.47 atm
1.3
58
W₂
WAIR
101.3 kPa
296 K
Pentane
469.8 K
845.64 R
33.3 atm
WVPL
Answer: D=
72
m
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 5 steps with 8 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemical-engineering and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall

Introduction to Chemical Engineering Thermodynami…
Chemical Engineering
ISBN:
9781259696527
Author:
J.M. Smith Termodinamica en ingenieria quimica, Hendrick C Van Ness, Michael Abbott, Mark Swihart
Publisher:
McGraw-Hill Education

Elementary Principles of Chemical Processes, Bind…
Chemical Engineering
ISBN:
9781118431221
Author:
Richard M. Felder, Ronald W. Rousseau, Lisa G. Bullard
Publisher:
WILEY

Elements of Chemical Reaction Engineering (5th Ed…
Chemical Engineering
ISBN:
9780133887518
Author:
H. Scott Fogler
Publisher:
Prentice Hall


Industrial Plastics: Theory and Applications
Chemical Engineering
ISBN:
9781285061238
Author:
Lokensgard, Erik
Publisher:
Delmar Cengage Learning

Unit Operations of Chemical Engineering
Chemical Engineering
ISBN:
9780072848236
Author:
Warren McCabe, Julian C. Smith, Peter Harriott
Publisher:
McGraw-Hill Companies, The