Now try and rearrange the E = hc/Aequation so that you can find the wavelength of a photon that has an energy of 5.15x 10-19 J. Type your answer here nm You are incorrect Hint When you do a wavelength calculation your wavelength must have units of meters to match the units of the speed of light! Fullscreen Resubmit
Now try and rearrange the E = hc/Aequation so that you can find the wavelength of a photon that has an energy of 5.15x 10-19 J. Type your answer here nm You are incorrect Hint When you do a wavelength calculation your wavelength must have units of meters to match the units of the speed of light! Fullscreen Resubmit
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter4: Introduction To Quantum Mechanics
Section: Chapter Questions
Problem 14P: Potassium atoms in a flame emit light as they undergo transitions from one energy level to another...
Related questions
Question
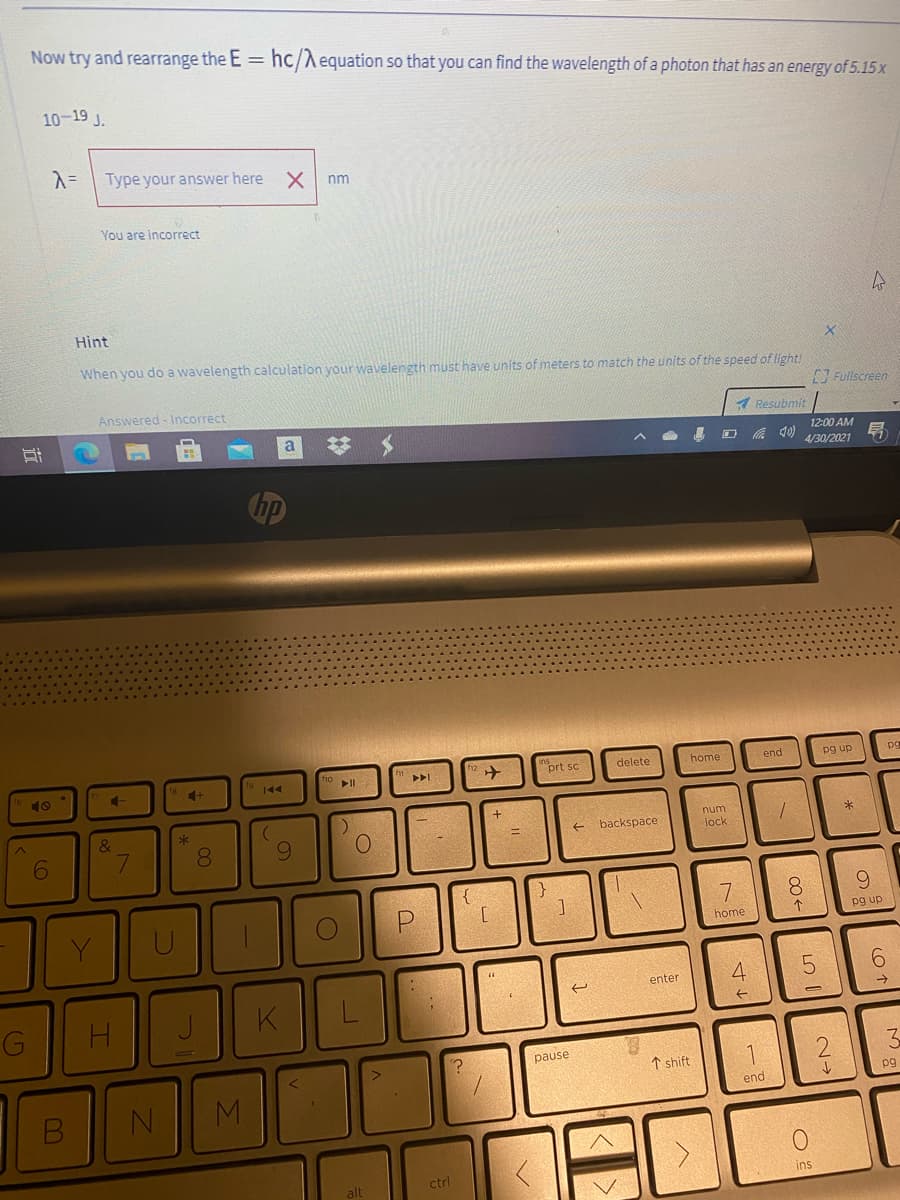
Transcribed Image Text:Now try and rearrange the E = hc/Aequation so that you can find the wavelength of a photon that has an energy of 5.15 x
10-19 J.
Type your answer here
nm
You are incorrect
Hint
When you do a wavelength calculation your wavelength must have units of meters to match the units of the speed of light!
Answered - Incorrect
Fullscreen
1 Resubmit
%23
12:00 AM
の
昂
4/30/2021
prt sc
delete
home
end
pg up
10
num
backspace
8.
lock
8.
6.
Y
home
pg up
4.
6.
enter
G
H.
K
pause
↑ shift
pg
end
alt
ctrl
ins
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning