O ACIDS AND BASES Identifying the major species in weak acid or weak base equilibria Ryan The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that HCH,CO, is a weak acid. O, acids: 0 ol 0.4 mol of NAOH is added to 0,0,.. 1.0 L of a 0.2 M HCH;CO2 O bases: CH,CO,OH 18 Ar solution. other: Na 0.58 mol of HI is added to acids: I 1.0 L of a solution that is 1.3 M in both HCH;CO2 bases: and KCH;CO,. other: Explanation Check 2020 McGraw-Hill Education. All Rights Reserved. Terms of Use | Privacy MacBook Pro 1II
O ACIDS AND BASES Identifying the major species in weak acid or weak base equilibria Ryan The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at equilibrium. You can leave out water itself. Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the formulas of the species that will act as neither acids nor bases in the 'other' row. You will find it useful to keep in mind that HCH,CO, is a weak acid. O, acids: 0 ol 0.4 mol of NAOH is added to 0,0,.. 1.0 L of a 0.2 M HCH;CO2 O bases: CH,CO,OH 18 Ar solution. other: Na 0.58 mol of HI is added to acids: I 1.0 L of a solution that is 1.3 M in both HCH;CO2 bases: and KCH;CO,. other: Explanation Check 2020 McGraw-Hill Education. All Rights Reserved. Terms of Use | Privacy MacBook Pro 1II
World of Chemistry, 3rd edition
3rd Edition
ISBN:9781133109655
Author:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Chapter16: Acids And Bases
Section: Chapter Questions
Problem 51A
Related questions
Question
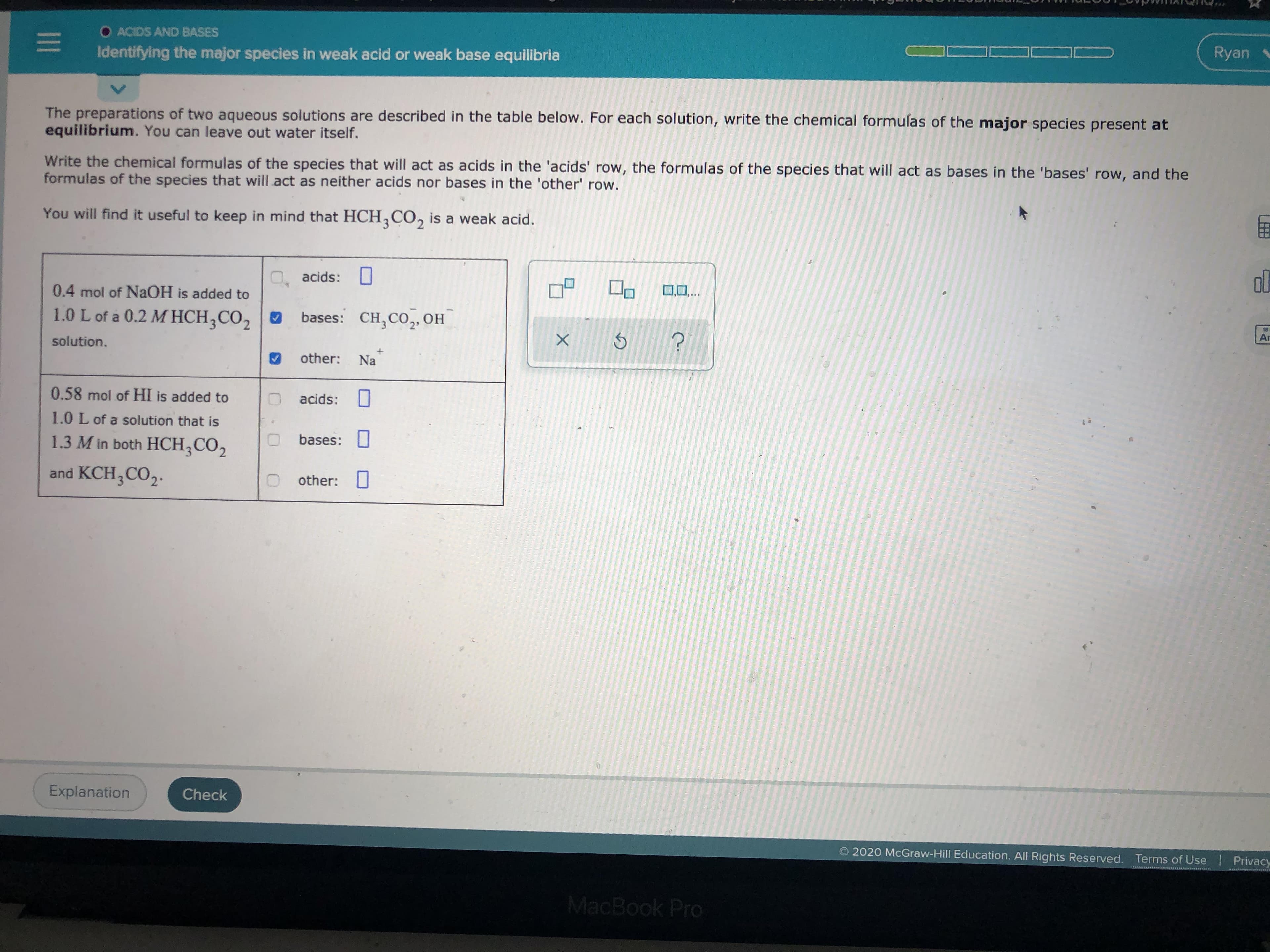
Transcribed Image Text:O ACIDS AND BASES
Identifying the major species in weak acid or weak base equilibria
Ryan
The preparations of two aqueous solutions are described in the table below. For each solution, write the chemical formulas of the major species present at
equilibrium. You can leave out water itself.
Write the chemical formulas of the species that will act as acids in the 'acids' row, the formulas of the species that will act as bases in the 'bases' row, and the
formulas of the species that will act as neither acids nor bases in the 'other' row.
You will find it useful to keep in mind that HCH,CO, is a weak acid.
O, acids: 0
ol
0.4 mol of NAOH is added to
0,0,..
1.0 L of a 0.2 M HCH;CO2 O
bases: CH,CO,OH
18
Ar
solution.
other:
Na
0.58 mol of HI is added to
acids: I
1.0 L of a solution that is
1.3 M in both HCH;CO2
bases:
and KCH;CO,.
other:
Explanation
Check
2020 McGraw-Hill Education. All Rights Reserved. Terms of Use | Privacy
MacBook Pro
1II
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 4 images

Recommended textbooks for you

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning


World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning