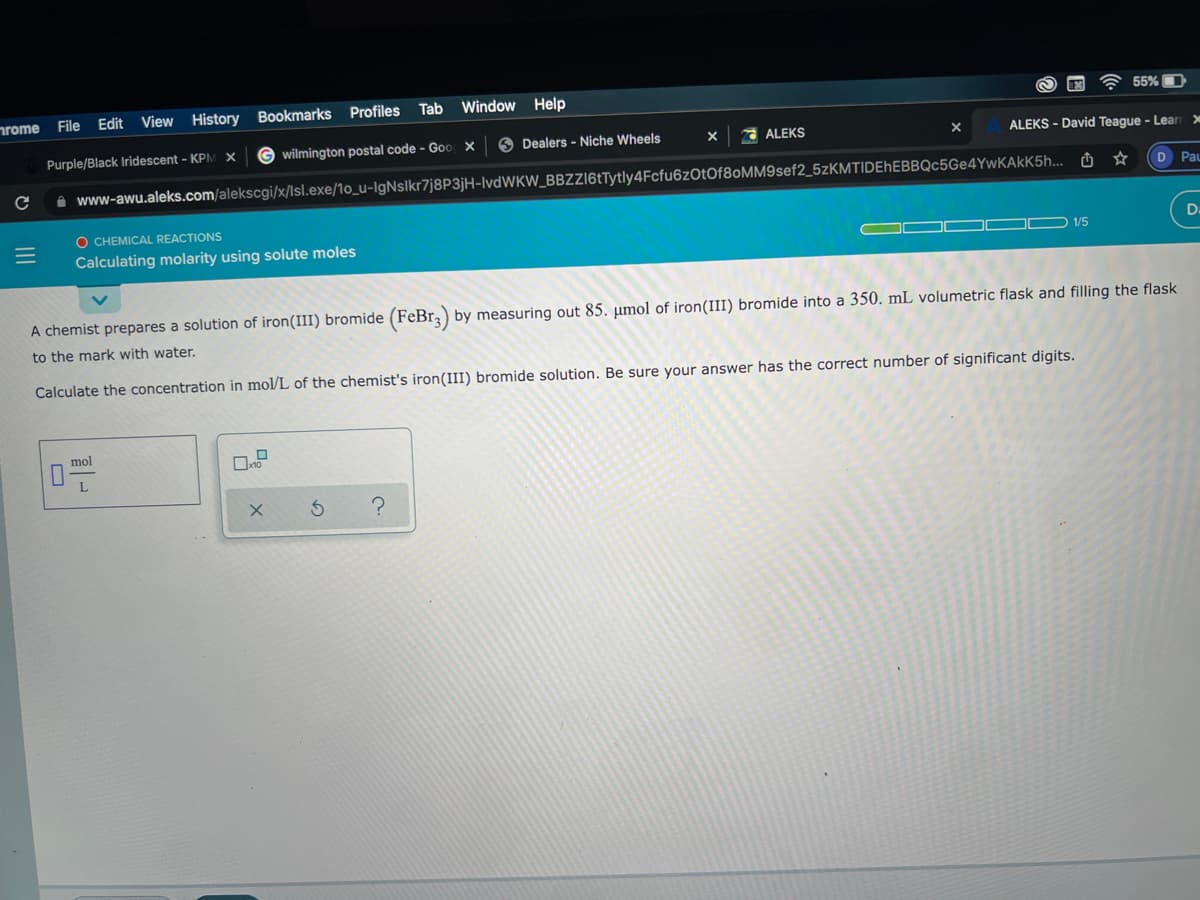
O CHEMICAL REACTIONS Calculating molarity using solute moles A chemist prepares a solution of iron(III) bromide (FeBr,) by measuring out 85. umol of iron(III) bromide into a 350. mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in mol/L of the chemist's iron(III) bromide solution. Be sure your answer has the correct number of significant digits. mol
O CHEMICAL REACTIONS Calculating molarity using solute moles A chemist prepares a solution of iron(III) bromide (FeBr,) by measuring out 85. umol of iron(III) bromide into a 350. mL volumetric flask and filling the flask to the mark with water. Calculate the concentration in mol/L of the chemist's iron(III) bromide solution. Be sure your answer has the correct number of significant digits. mol
Chemistry & Chemical Reactivity
10th Edition
ISBN:9781337399074
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter3: Chemical Reactions
Section3.9: Classifying Reactions In Aqueous Solution
Problem 2.2ACP
Related questions
Question
Transcribed Image Text:nrome
File
Edit
View History Bookmarks
Profiles
Tab
Window Help
55%
Purple/Black Iridescent - KPM X
G wilmington postal code - Goo X
O Dealers - Niche Wheels
a ALEKS
ALEKS - David Teague - Lear
A www-awu.aleks.com/alekscgi/x/lsl.exe/1o_u-IgNslkr7j8P3jH-IvdWKW_BBZZI6tTytly4Fcfu6zOtOf8oMM9sef2_5zKMTIDEhEBBQc5Ge4YwKAkK5h... O *
D PaL
O CHEMICAL REACTIONS
D.
Calculating molarity using solute moles
O 1/5
A chemist prepares a solution of iron(III) bromide (FeBr, by measuring out 85. µmol of iron(III) bromide into a 350. mL volumetric flask and filling the flask
to the mark with water.
Calculate the concentration in mol/L of the chemist's iron(III) bromide solution. Be sure your answer has the correct number of significant digits.
mol
II
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning