O ENTROPY AND FREE ENERGY Calculating entropy change using the Boltzmann hypothesis DE Two nitro (NO, groups are chemically bonded to a patch of surface. They can't move to another location on the surface, but they can rotate (see sketch at right). It turns out that the amount of rotational kinetic energy each NO, group can have is required to be a multiple of &, where -24 J. In other words, each NO, group could have & of rotational kinetic energy, or 2&, or 3, and so forth -but it =1.0 x 10 cannot have just any old amount of rotational kinetic energy. Two rotating NO, groups Suppose the total rotational kinetic energy in this system is initially known to be 100E. Then, some heat is removed from the bonded to a surface. system, and he total rotational kinetic energy falls to 75&. Calculate the change in entropy. Round your answer to 3 significant digits, and be sure it has the correct unit symbol. x10 uD Explanation Check 2019 McGraw-Hill Education. All Rights Reserved. Terms of Use Priv X II
O ENTROPY AND FREE ENERGY Calculating entropy change using the Boltzmann hypothesis DE Two nitro (NO, groups are chemically bonded to a patch of surface. They can't move to another location on the surface, but they can rotate (see sketch at right). It turns out that the amount of rotational kinetic energy each NO, group can have is required to be a multiple of &, where -24 J. In other words, each NO, group could have & of rotational kinetic energy, or 2&, or 3, and so forth -but it =1.0 x 10 cannot have just any old amount of rotational kinetic energy. Two rotating NO, groups Suppose the total rotational kinetic energy in this system is initially known to be 100E. Then, some heat is removed from the bonded to a surface. system, and he total rotational kinetic energy falls to 75&. Calculate the change in entropy. Round your answer to 3 significant digits, and be sure it has the correct unit symbol. x10 uD Explanation Check 2019 McGraw-Hill Education. All Rights Reserved. Terms of Use Priv X II
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter13: Spontaneous Processes And Thermodynamic Equilibrium
Section: Chapter Questions
Problem 49AP
Related questions
Question
Calculate the change in entropy Roger answer to three significant digits and be sure he has the crate in at
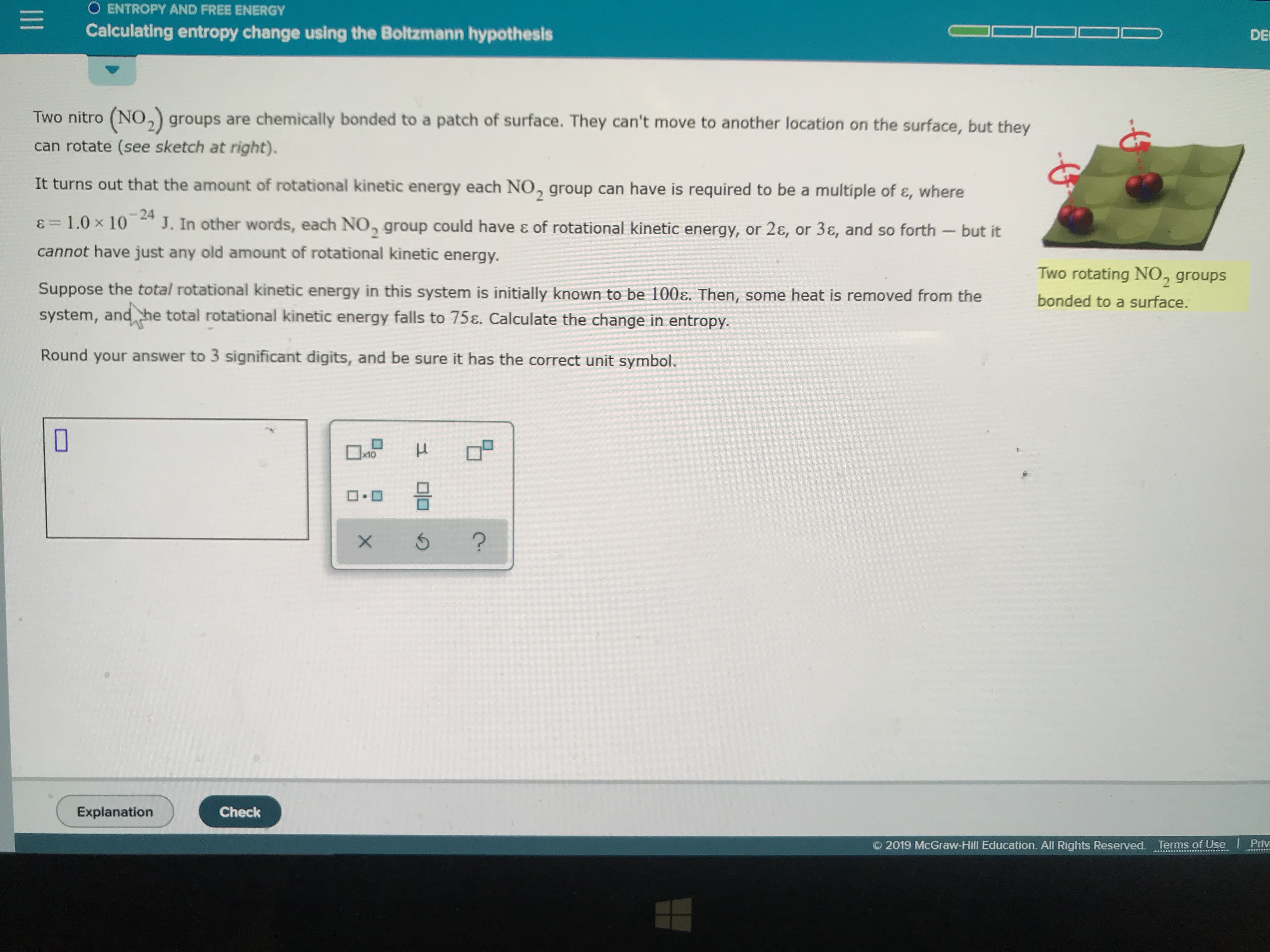
Transcribed Image Text:O ENTROPY AND FREE ENERGY
Calculating entropy change using the Boltzmann hypothesis
DE
Two nitro (NO, groups are chemically bonded to a patch of surface. They can't move to another location on the surface, but they
can rotate (see sketch at right).
It turns out that the amount of rotational kinetic energy each NO, group can have is required to be a multiple of &, where
-24
J. In other words, each NO, group could have & of rotational kinetic energy, or 2&, or 3, and so forth -but it
=1.0 x 10
cannot have just any old amount of rotational kinetic energy.
Two rotating NO, groups
Suppose the total rotational kinetic energy in this system is initially known to be 100E. Then, some heat is removed from the
bonded to a surface.
system, and he total rotational kinetic energy falls to 75&. Calculate the change in entropy.
Round your answer to 3 significant digits, and be sure it has the correct unit symbol.
x10
uD
Explanation
Check
2019 McGraw-Hill Education. All Rights Reserved. Terms of Use
Priv
X
II
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
