1. Draw the two Watson-Crick Bases Pairs and show how G- C and A-T recognize each other by hydrogen bonding. 2. Draw the full structure of two nucleotides - one from DNA and one from RNA. Point out the key difference between the two. 3. Write out an example of a condensation reaction forming a phosphate-sugar bond. 4. Write out an example of a condensation reaction between a nucleic acid base and a sugar. 5. State the "Central Dogma" of biochemistry.
1. Draw the two Watson-Crick Bases Pairs and show how G- C and A-T recognize each other by hydrogen bonding. 2. Draw the full structure of two nucleotides - one from DNA and one from RNA. Point out the key difference between the two. 3. Write out an example of a condensation reaction forming a phosphate-sugar bond. 4. Write out an example of a condensation reaction between a nucleic acid base and a sugar. 5. State the "Central Dogma" of biochemistry.
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter28: Nucleic Acids
Section: Chapter Questions
Problem 28.37P
Related questions
Question
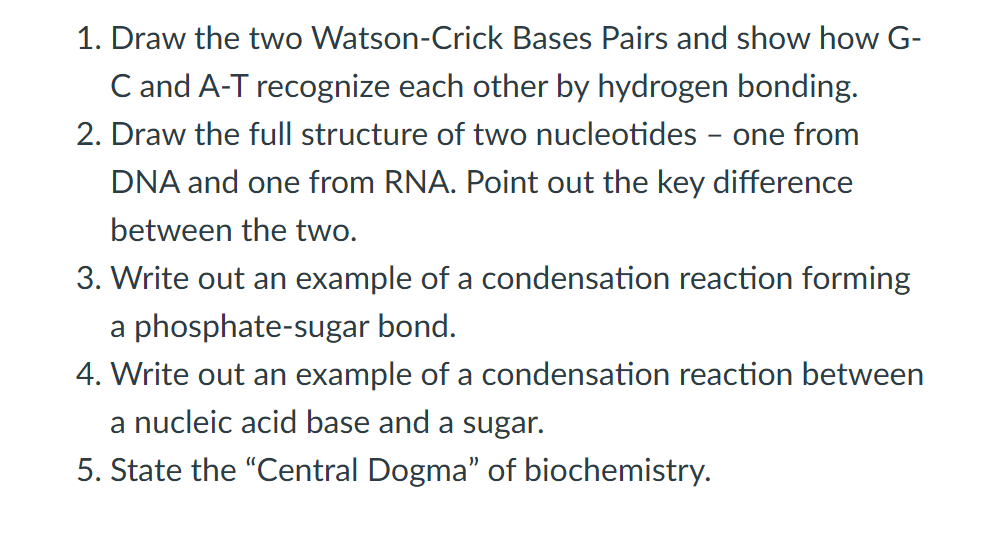
Transcribed Image Text:1. Draw the two Watson-Crick Bases Pairs and show how G-
C and A-T recognize each other by hydrogen bonding.
2. Draw the full structure of two nucleotides - one from
DNA and one from RNA. Point out the key difference
between the two.
3. Write out an example of a condensation reaction forming
a phosphate-sugar bond.
4. Write out an example of a condensation reaction between
a nucleic acid base and a sugar.
5. State the "Central Dogma" of biochemistry.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

General Chemistry - Standalone book (MindTap Cour…
Chemistry
ISBN:
9781305580343
Author:
Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning