O Previous Page 8 of 16 Next O Subm Silicon carbide, SiC, is one of the hardest materials known Surpassed in hardness only by diamond, it is sometimes known commercially as carborundum. Silicon carbide is used primarily as an abrasive for sandpaper and is manufactured by heating common sand (silicon dioxide, SiO2) with carbon in a furnace SiO, (a) + C(s) - CO(g) + SiC(3) What mass of silicon carbide should result when 4.3 kg of pure sand is heated with an excess of carbon? Mass kg
O Previous Page 8 of 16 Next O Subm Silicon carbide, SiC, is one of the hardest materials known Surpassed in hardness only by diamond, it is sometimes known commercially as carborundum. Silicon carbide is used primarily as an abrasive for sandpaper and is manufactured by heating common sand (silicon dioxide, SiO2) with carbon in a furnace SiO, (a) + C(s) - CO(g) + SiC(3) What mass of silicon carbide should result when 4.3 kg of pure sand is heated with an excess of carbon? Mass kg
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter23: Potentiometry
Section: Chapter Questions
Problem 23.12QAP: What arc the advantages of microfabricated ISEs? Describe typical applications of this type of...
Related questions
Question
Transcribed Image Text:My Home
* OWLV2 | Online teaching and lea x
a cvg.cengagenów.com/ilm/takeAssignment/takeCXPCompliantActivity.do?locator=assignment-take
Sa
9- CHEMICAL QUANTITIES
Study
Pr
O Previous Page 8 of 16 Next O
Subni
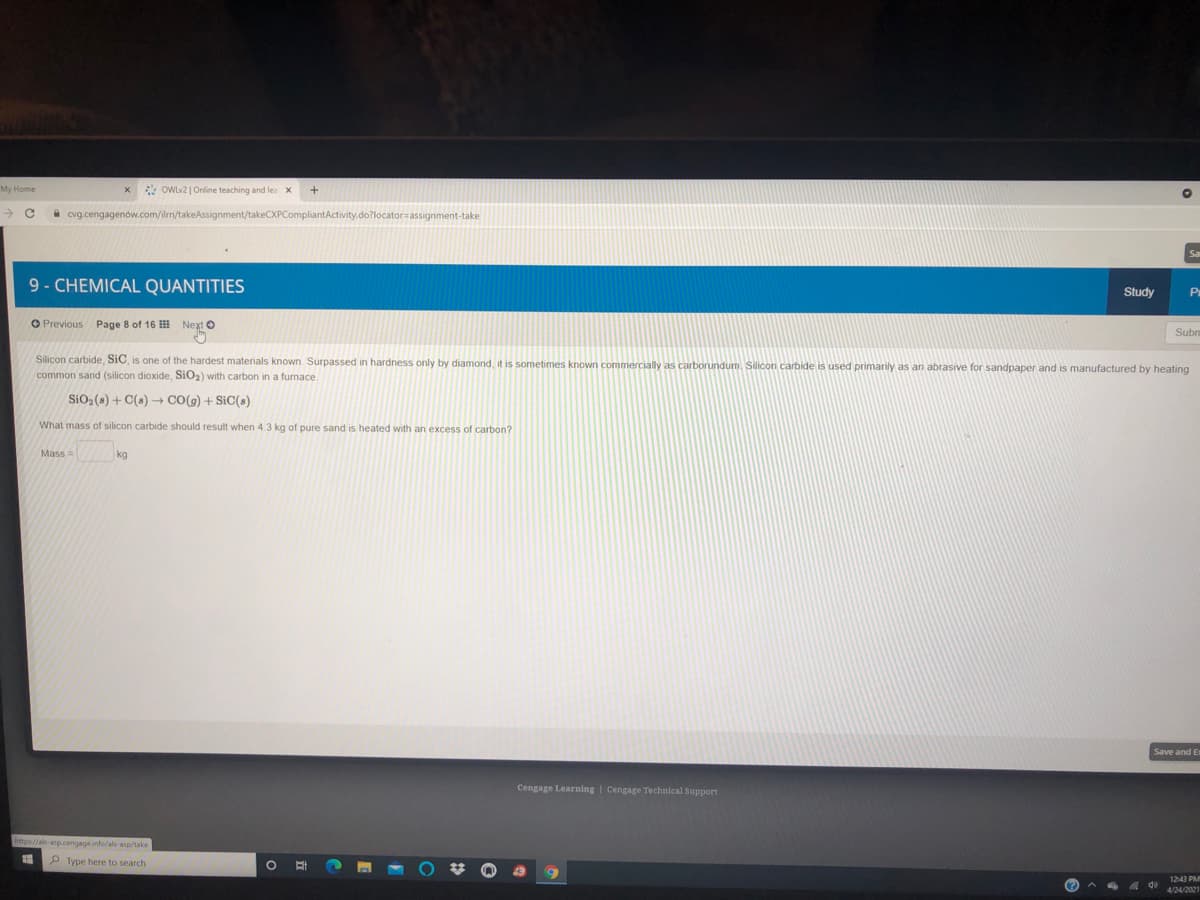
Silicon carbide, SiC, is one of the hardest materials known. Surpassed in hardness only by diamond, it is sometimes known commercially as carborundum. Silicon carbide is used primarily as an abrasive for sandpaper and is manufactured by heating
common sand (silicon dioxide, SiO2) with carbon in a furnace.
SiO2 (s) + C(s) + CO(g) + SiC(s)
What mass of silicon carbide should result when 4.3 kg of pure sand is heated with an excess of carbon?
Mass =
kg
Save and Er
Cengage Learning | Cengage Technical Support
https://als-anp.cengage.info/als-asp/take
P Type here to search
1243 PM
4/24/2021
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning