#of Protons Group Hof Valence Ion that Will Form Element Oxidation Number Electrons Lithium #3 #3 23 Add Here! Sodium #3 #3 #3 # Add Here! Beryllium #3 #3 #3 #3 Add Here! Aluminum #3 #3 #3 23 Add Here! Phosphorous #3 #3 #3 23 Add Here! Oxygen # #3 #3 # Add Here! Fluorine #3 #3 Add Here! 2. Which clements above will form cations? List them below. Add Text Here! 3. Which clements above will form anions? List them below. Add Text Here! 4. What does aluminum neod to do in order to become stable? Add Text Here! %23 %23
#of Protons Group Hof Valence Ion that Will Form Element Oxidation Number Electrons Lithium #3 #3 23 Add Here! Sodium #3 #3 #3 # Add Here! Beryllium #3 #3 #3 #3 Add Here! Aluminum #3 #3 #3 23 Add Here! Phosphorous #3 #3 #3 23 Add Here! Oxygen # #3 #3 # Add Here! Fluorine #3 #3 Add Here! 2. Which clements above will form cations? List them below. Add Text Here! 3. Which clements above will form anions? List them below. Add Text Here! 4. What does aluminum neod to do in order to become stable? Add Text Here! %23 %23
Biochemistry
9th Edition
ISBN:9781319114671
Author:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Chapter1: Biochemistry: An Evolving Science
Section: Chapter Questions
Problem 1P
Related questions
Question
100%
This is NOT graded. Do # 2,3 and 4
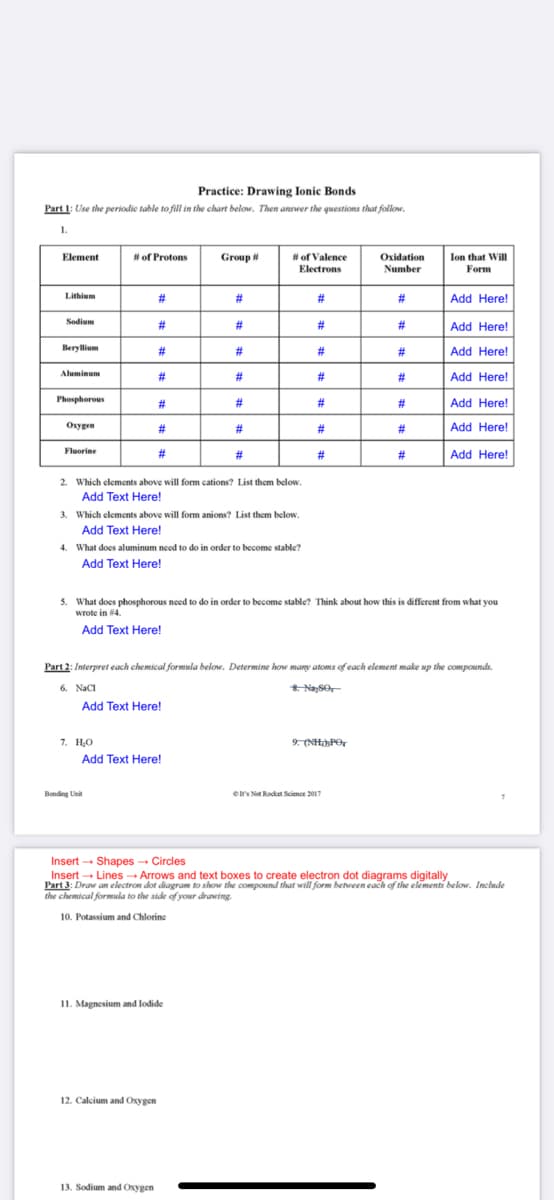
Transcribed Image Text:Practice: Drawing Ionic Bonds
Part 1: Use the periodic table to fill in the chart below. Then answer the questions that follow.
1.
Ion that Wil
Form
Element
# of Protons
Group #
# of Valence
Electrons
Oxidation
Number
Lithium
#3
%23
#3
#3
Add Here!
Sodium
#3
23
#3
#3
Add Here!
Beryllium
#3
#3
%23
#3
#3
Add Here!
Aluminum
#3
23
#3
#3
Add Here!
Phosphorous
#3
#3
#3
#3
Add Here!
Oxygen
#3
#
#3
#3
Add Here!
Fluorine
2#
%23
#3
#3
Add Here!
2. Which elements above will form cations? List them below.
Add Text Here!
3. Which elements above will form anions? List them below.
Add Text Here!
4. What does aluminum need to do in order to become stable?
Add Text Here!
5. What does phosphorous need to do in order to become stable? Think about how this is different from what you
wrote in #4.
Add Text Here!
Part 2: Interpret each chemical formela below. Determine how many atoms of each element make up the compounds.
6. NacI
8 NaSO
Add Text Here!
7. H,O
9. (NH»PO,
Add Text Here!
Bonding Unit
Os Not Rocket Science 2017
Insert - Shapes - Circles
Insert - Lines→ Arrows and text boxes to create electron dot diagrams digitally
Part 3: Draw an electron dot diagram to show the compound that will form between each of the elements below. Inchude
the chemical formsela to the side of your drawing.
10. Potassium and Chlorine
11. Magnesium and lodide
12. Calcium and Oxygen
13. Sodium and Oxygen
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Recommended textbooks for you

Biochemistry
Biochemistry
ISBN:
9781319114671
Author:
Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:
W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:
9781464126116
Author:
David L. Nelson, Michael M. Cox
Publisher:
W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul…
Biochemistry
ISBN:
9781118918401
Author:
Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:
WILEY

Biochemistry
Biochemistry
ISBN:
9781319114671
Author:
Lubert Stryer, Jeremy M. Berg, John L. Tymoczko, Gregory J. Gatto Jr.
Publisher:
W. H. Freeman

Lehninger Principles of Biochemistry
Biochemistry
ISBN:
9781464126116
Author:
David L. Nelson, Michael M. Cox
Publisher:
W. H. Freeman

Fundamentals of Biochemistry: Life at the Molecul…
Biochemistry
ISBN:
9781118918401
Author:
Donald Voet, Judith G. Voet, Charlotte W. Pratt
Publisher:
WILEY

Biochemistry
Biochemistry
ISBN:
9781305961135
Author:
Mary K. Campbell, Shawn O. Farrell, Owen M. McDougal
Publisher:
Cengage Learning

Biochemistry
Biochemistry
ISBN:
9781305577206
Author:
Reginald H. Garrett, Charles M. Grisham
Publisher:
Cengage Learning

Fundamentals of General, Organic, and Biological …
Biochemistry
ISBN:
9780134015187
Author:
John E. McMurry, David S. Ballantine, Carl A. Hoeger, Virginia E. Peterson
Publisher:
PEARSON