One kg of air occupies 0.048 m at 12.5 bar and 537 C° it is expanded at constant temperature to a final volume of 0.336 m³. Calculate the pressure at the end of expansion and the work done during the expansion?
One kg of air occupies 0.048 m at 12.5 bar and 537 C° it is expanded at constant temperature to a final volume of 0.336 m³. Calculate the pressure at the end of expansion and the work done during the expansion?
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter1: Gases And The Zeroth Law Of Thermodynamics
Section: Chapter Questions
Problem 1.66E
Related questions
Question
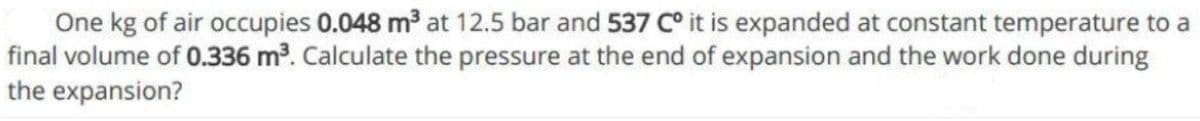
Transcribed Image Text:One kg of air occupies 0.048 m at 12.5 bar and 537 C° it is expanded at constant temperature to a
final volume of 0.336 m³. Calculate the pressure at the end of expansion and the work done during
the expansion?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning