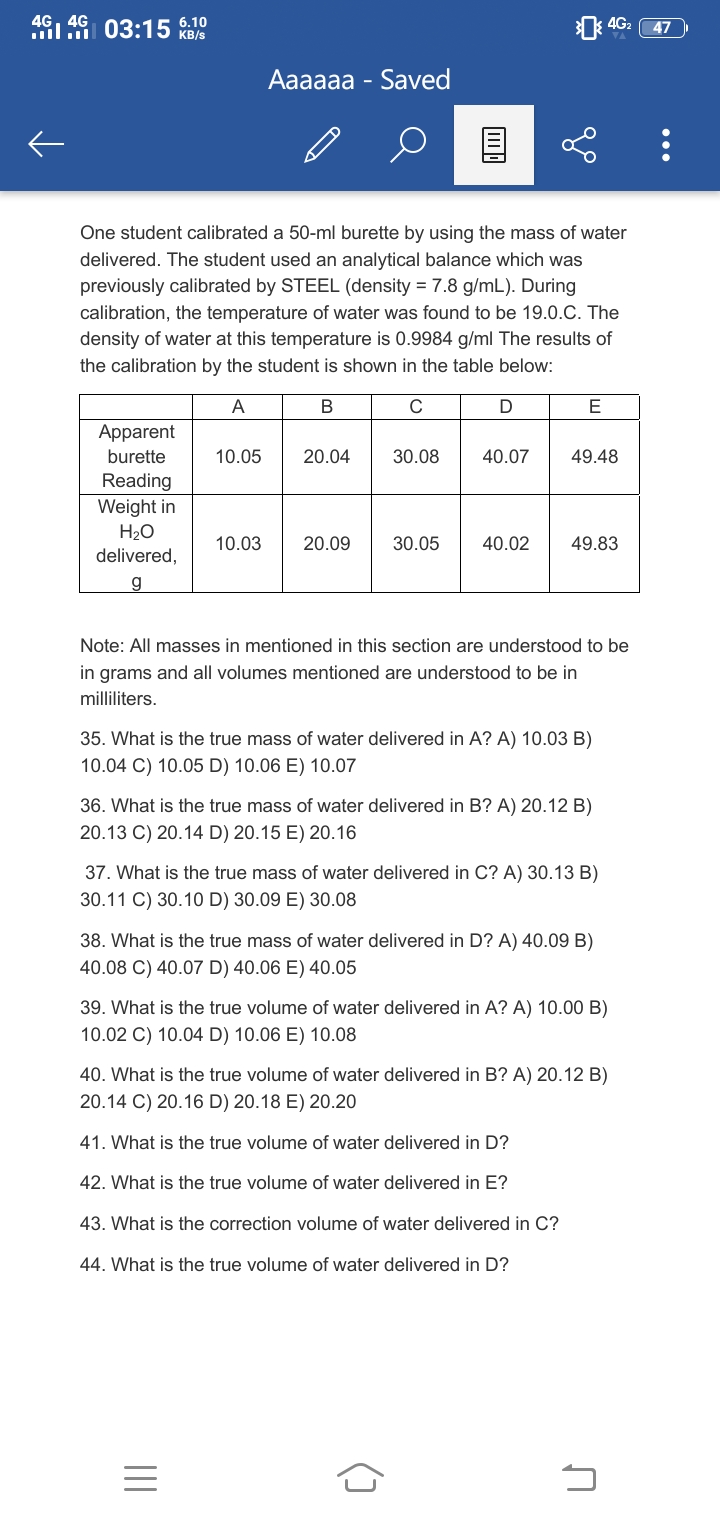
One student calibrated a 50-ml burette by using the mass of water delivered. The student used an analytical balance which was previously calibrated by STEEL (density = 7.8 g/mL). During calibration, the temperature of water was found to be 19.0.C. The density of water at this temperature is 0.9984 g/ml The results of the calibration by the student is shown in the table below: A B C D E Apparent burette reading,mL 10.05 20.04 30.08 40.07 49.98 Weight H2O delivered, g 10.03 20.09 30.05 40.02 49.83 Note: All masses in mentioned in this section are understood to be in grams and all volumes mentioned are understood to be in milliliters. 35. What is the true mass of water delivered in A? A) 10.03 B) 10.04 C) 10.05 D) 10.06 E) 10.07 36. What is the true mass of water delivered in B? A) 20.12 B) 20.13 C) 20.14 D) 20.15 E) 20.16 37. What is the true mass of water delivered in C? A) 30.13 B) 30.11 C) 30.10 D) 30.09 E) 30.08
One student calibrated a 50-ml burette by using the mass of water delivered. The student used an analytical balance which was previously calibrated by STEEL (density = 7.8 g/mL). During calibration, the temperature of water was found to be 19.0.C. The density of water at this temperature is 0.9984 g/ml The results of the calibration by the student is shown in the table below:
| A | B | C | D | E | |
| Apparent burette reading,mL | 10.05 | 20.04 | 30.08 | 40.07 | 49.98 |
| Weight H2O delivered, g | 10.03 | 20.09 | 30.05 | 40.02 | 49.83 |
Note: All masses in mentioned in this section are understood to be in grams and all volumes mentioned are understood to be in milliliters.
35. What is the true mass of water delivered in A? A) 10.03 B) 10.04 C) 10.05 D) 10.06 E) 10.07
36. What is the true mass of water delivered in B? A) 20.12 B) 20.13 C) 20.14 D) 20.15 E) 20.16
37. What is the true mass of water delivered in C? A) 30.13 B) 30.11 C) 30.10 D) 30.09 E) 30.08
38. What is the true mass of water delivered in D? A) 40.09 B) 40.08 C) 40.07 D) 40.06 E) 40.05 39. What is the true volume of water delivered in A? A) 10.00 B) 10.02 C) 10.04 D) 10.06 E) 10.08
40. What is the true volume of water delivered in B? A) 20.12 B) 20.14 C) 20.16 D) 20.18 E) 20.20
Trending now
This is a popular solution!
Step by step
Solved in 7 steps with 5 images









