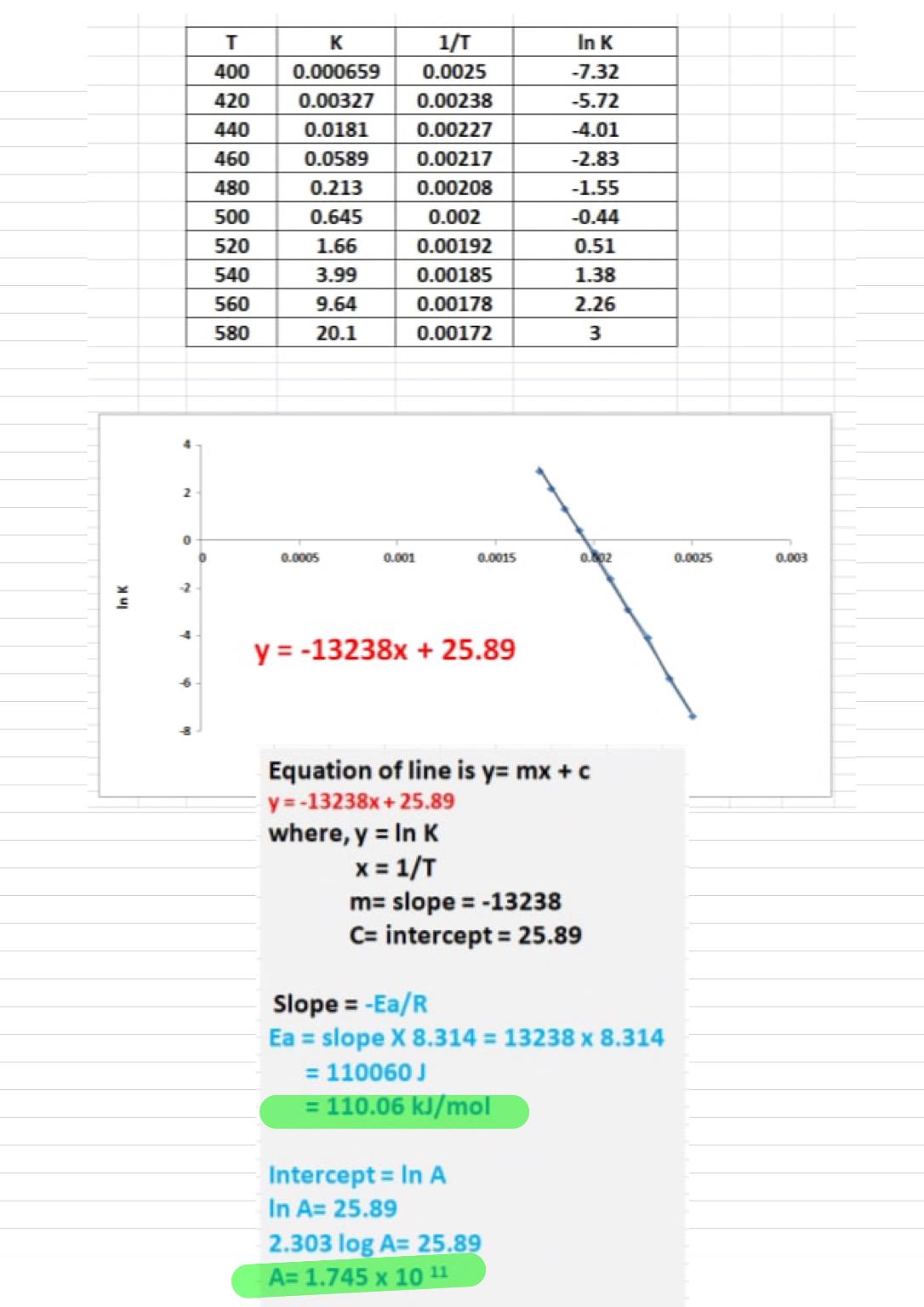
onstant (?) data collected during the experiments. Plot the data to answer the questions. What is the value of the activation energy, ?a , for this reaction? ? (?) ? (?−1) 400 0.000659 420 0.00327 440 0.0181 460 0.0589 480 0.213 500 0.615 520 1.66 540 3.99 560 9.64 580 20.1 Ea= What is the value of the pre‑exponential factor (sometimes called the frequency factor), ? , for this reaction?
onstant (?) data collected during the experiments. Plot the data to answer the questions. What is the value of the activation energy, ?a , for this reaction? ? (?) ? (?−1) 400 0.000659 420 0.00327 440 0.0181 460 0.0589 480 0.213 500 0.615 520 1.66 540 3.99 560 9.64 580 20.1 Ea= What is the value of the pre‑exponential factor (sometimes called the frequency factor), ? , for this reaction?
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter11: Chemical Kinetics: Rates Of Reactions
Section11.2: Effect Of Concentration On Reaction Rate
Problem 11.3PSP
Related questions
Question
The rate of a certain reaction was studied at various temperatures. The table shows temperature (?) and rate constant (?) data collected during the experiments. Plot the data to answer the questions.
What is the value of the activation energy, ?a , for this reaction?
| ? (?) | ? (?−1) |
|---|---|
| 400 | 0.000659 |
| 420 | 0.00327 |
| 440 | 0.0181 |
| 460 | 0.0589 |
| 480 | 0.213 |
| 500 | 0.615 |
| 520 | 1.66 |
| 540 | 3.99 |
| 560 | 9.64 |
| 580 | 20.1 |
Ea=
What is the value of the pre‑exponential factor (sometimes called the frequency factor), ? , for this reaction?
Expert Solution
Step 1
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,