or the following amino acids: Isoleucine and Tyrosine a. Draw its complete protonic equilibria. Indicate the net charge of each form and encircle the zwitterionic form. Provide labels (A, B, C, etc.) for each form. b. Calculate the IpH of the amino acid. c. At what pH range(s) it can act as a buffer?
or the following amino acids: Isoleucine and Tyrosine a. Draw its complete protonic equilibria. Indicate the net charge of each form and encircle the zwitterionic form. Provide labels (A, B, C, etc.) for each form. b. Calculate the IpH of the amino acid. c. At what pH range(s) it can act as a buffer?
Chapter9: Parenteral Medication Labels And Dosage Calculation
Section: Chapter Questions
Problem 3.2P
Related questions
Question
For the following amino acids: Isoleucine and Tyrosine
a. Draw its complete protonic equilibria. Indicate the net charge of each form and
encircle the zwitterionic form. Provide labels (A, B, C, etc.) for each form.
b. Calculate the IpH of the amino acid.
c. At what pH range(s) it can act as a buffer?
d. What will be the predominant form(s) of the amino acid at pH (i) 1.5; (ii) 3.0; (iii) 4.5;
(iv) 6.0; (v) 7.5 and (vi) 9.0?
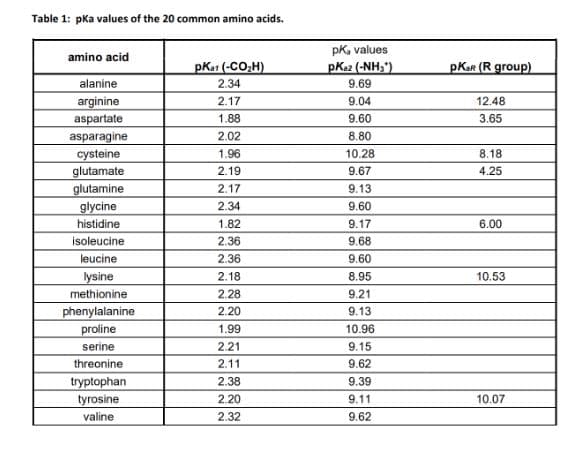
Transcribed Image Text:Table 1: pka values of the 20 common amino acids.
amino acid
alanine
arginine
aspartate
asparagine
cysteine
glutamate
glutamine
glycine
histidine
isoleucine
leucine
lysine
methionine
phenylalanine
proline
serine
threonine
tryptophan
tyrosine
valine
pka1 (-CO₂H)
2.34
2.17
1.88
2.02
1.96
2.19
2.17
2.34
1.82
2.36
2.36
2.18
2.28
2.20
1.99
2.21
2.11
2.38
2.20
2.32
pk, values
PK82 (-NH₂)
9.69
9.04
9.60
8.80
10.28
9.67
9.13
9.60
9.17
9.68
9.60
8.95
9.21
9.13
10.96
9.15
9.62
9.39
9.11
9.62
pKar (R group)
12.48
3.65
8.18
4.25
6.00
10.53
10.07
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 2 images

Follow-up Questions
Read through expert solutions to related follow-up questions below.
Follow-up Question
d. What will be the predominant form(s) of the amino acid at pH (i) 1.5; (ii) 3.0; (iii) 4.5; (iv) 6.0; (v) 7.5 and (vi) 9.0?
Solution
Recommended textbooks for you


Biology: The Dynamic Science (MindTap Course List)
Biology
ISBN:
9781305389892
Author:
Peter J. Russell, Paul E. Hertz, Beverly McMillan
Publisher:
Cengage Learning


Biology: The Dynamic Science (MindTap Course List)
Biology
ISBN:
9781305389892
Author:
Peter J. Russell, Paul E. Hertz, Beverly McMillan
Publisher:
Cengage Learning