Organic Chemistry Chapter Five Synthesis of Alkenes The first step in the mechanism of this reaction is a rapid and reversible protonation of the alcohol by the acid, followed in the second step by a rate-determining loss of water to form a tertiary carbocation. This process is illustrated here for 3-methyl-3-pentanol. Н +OH + H2PO, :ÖH H-0-P-OH ОН 3-methyl-3-pentanol ОН + H20 - H20 In the third step, the carbocation loses a proton to give a mixture of alkenes. - H+ alkene mixture In this experiment, there are several different alkenes that can be formed. However, one of the alkenes often predominates. In Part A of this experiment, the alcohol to be dehydrated is 3-methyl-3-pentanol, while in Part B, the alcohol will be 3,3-dimethyl-2-butanol. The product compositions will be evaluated by gas chromatography. ОН H,PO4 alkene products In Part C, you will dehydrate an unknown alcohol. The two possible unknown alcohols are 2-methyl-1-phenyl-2-propanol or 2-phenyl-2-butanol. Based upon the number of alkenes formed in the reaction, you will identify the structure of the unknown alcohol. You will then identify the predominant alkene through analysis of the product mixture by gas chromatography (GC) or gas chromatography-mass spectrometry (GC-MS). ОН ОН 2-methyl-1-phenyl-2-propanol 2-phenyl-2-butanol Prelab Assignment 1. Parts A and B: Draw the structures of all of the alkenes that could be formed during the assigned experiment. Provide an IUPAC name for each possible product. 2. Predict which alkene should be the major product. 3. Complete the mechanism, showing the loss of a proton that generates each alkene that could be formed from the assigned reaction. 4. Part C: Draw the structures of the alkenes that could be formed from each of the two possible alcohols. Provide an IUPAC name for each possible product.
Organic Chemistry Chapter Five Synthesis of Alkenes The first step in the mechanism of this reaction is a rapid and reversible protonation of the alcohol by the acid, followed in the second step by a rate-determining loss of water to form a tertiary carbocation. This process is illustrated here for 3-methyl-3-pentanol. Н +OH + H2PO, :ÖH H-0-P-OH ОН 3-methyl-3-pentanol ОН + H20 - H20 In the third step, the carbocation loses a proton to give a mixture of alkenes. - H+ alkene mixture In this experiment, there are several different alkenes that can be formed. However, one of the alkenes often predominates. In Part A of this experiment, the alcohol to be dehydrated is 3-methyl-3-pentanol, while in Part B, the alcohol will be 3,3-dimethyl-2-butanol. The product compositions will be evaluated by gas chromatography. ОН H,PO4 alkene products In Part C, you will dehydrate an unknown alcohol. The two possible unknown alcohols are 2-methyl-1-phenyl-2-propanol or 2-phenyl-2-butanol. Based upon the number of alkenes formed in the reaction, you will identify the structure of the unknown alcohol. You will then identify the predominant alkene through analysis of the product mixture by gas chromatography (GC) or gas chromatography-mass spectrometry (GC-MS). ОН ОН 2-methyl-1-phenyl-2-propanol 2-phenyl-2-butanol Prelab Assignment 1. Parts A and B: Draw the structures of all of the alkenes that could be formed during the assigned experiment. Provide an IUPAC name for each possible product. 2. Predict which alkene should be the major product. 3. Complete the mechanism, showing the loss of a proton that generates each alkene that could be formed from the assigned reaction. 4. Part C: Draw the structures of the alkenes that could be formed from each of the two possible alcohols. Provide an IUPAC name for each possible product.
Organic Chemistry
8th Edition
ISBN:9781305580350
Author:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Chapter24: Catalytic Carbon-carbon Bond Formation
Section: Chapter Questions
Problem 24.36P
Related questions
Question
Problems 1 and 3 for part a (3-methyl-3-pentanol with phosphoric acid)
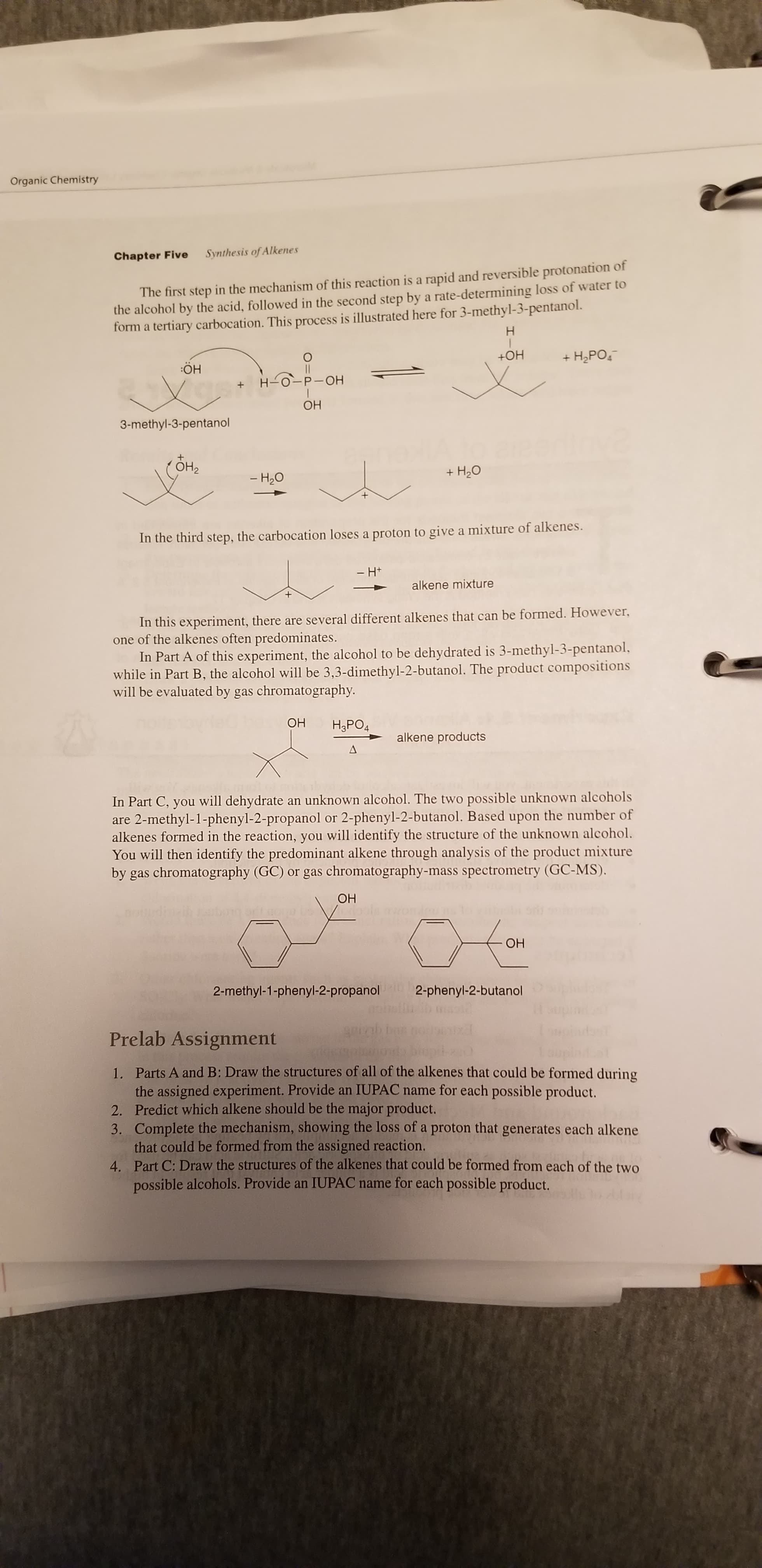
Transcribed Image Text:Organic Chemistry
Chapter Five
Synthesis of Alkenes
The first step in the mechanism of this reaction is a rapid and reversible protonation of
the alcohol by the acid, followed in the second step by a rate-determining loss of water to
form a tertiary carbocation. This process is illustrated here for 3-methyl-3-pentanol.
Н
+OH
+ H2PO,
:ÖH
H-0-P-OH
ОН
3-methyl-3-pentanol
ОН
+ H20
- H20
In the third step, the carbocation loses a proton to give a mixture of alkenes.
- H+
alkene mixture
In this experiment, there are several different alkenes that can be formed. However,
one of the alkenes often predominates.
In Part A of this experiment, the alcohol to be dehydrated is 3-methyl-3-pentanol,
while in Part B, the alcohol will be 3,3-dimethyl-2-butanol. The product compositions
will be evaluated by gas chromatography.
ОН
H,PO4
alkene products
In Part C, you will dehydrate an unknown alcohol. The two possible unknown alcohols
are 2-methyl-1-phenyl-2-propanol or 2-phenyl-2-butanol. Based upon the number of
alkenes formed in the reaction, you will identify the structure of the unknown alcohol.
You will then identify the predominant alkene through analysis of the product mixture
by gas chromatography (GC) or gas chromatography-mass spectrometry (GC-MS).
ОН
ОН
2-methyl-1-phenyl-2-propanol
2-phenyl-2-butanol
Prelab Assignment
1. Parts A and B: Draw the structures of all of the alkenes that could be formed during
the assigned experiment. Provide an IUPAC name for each possible product.
2. Predict which alkene should be the major product.
3. Complete the mechanism, showing the loss of a proton that generates each alkene
that could be formed from the assigned reaction.
4. Part C: Draw the structures of the alkenes that could be formed from each of the two
possible alcohols. Provide an IUPAC name for each possible product.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 3 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning