Osmosis occurs when two solutions of different concentrations are separated by a semipermeable membrane. The membrane allows solvent particles, but X Incorrect; Try Again; 5 attempts remaining not solute particles, to pass through. Thus, the solvent moves moves toward the solution of higher concentration (to dilute the two solutions have the it) until concentration, same Part B or until gravity overtakes the osmotic pressure, II. The osmotic pressure between a solution and pure solvent depends on the number of solute particles and thus is a colligative property. It can be expressed as Osmosis is the process responsible for carrying nutrients and water from groundwater supplies to the upper parts of trees. The osmotic pressures required for this process can be as high as 20.1 bar . What would the molar concentration of the tree sap have to be to achieve this pressure on a day when the temperature is 24 °C ? II = MRT Express your answer to three significant figures and include the appropriate units. where M is the total molarity of the solute particles, R= 0.08314 L bar mol¯1 K¯' is the gas constant, and T is the Kelvin temperature. • View Available Hint(s) HÀ ? Value Units
Osmosis occurs when two solutions of different concentrations are separated by a semipermeable membrane. The membrane allows solvent particles, but X Incorrect; Try Again; 5 attempts remaining not solute particles, to pass through. Thus, the solvent moves moves toward the solution of higher concentration (to dilute the two solutions have the it) until concentration, same Part B or until gravity overtakes the osmotic pressure, II. The osmotic pressure between a solution and pure solvent depends on the number of solute particles and thus is a colligative property. It can be expressed as Osmosis is the process responsible for carrying nutrients and water from groundwater supplies to the upper parts of trees. The osmotic pressures required for this process can be as high as 20.1 bar . What would the molar concentration of the tree sap have to be to achieve this pressure on a day when the temperature is 24 °C ? II = MRT Express your answer to three significant figures and include the appropriate units. where M is the total molarity of the solute particles, R= 0.08314 L bar mol¯1 K¯' is the gas constant, and T is the Kelvin temperature. • View Available Hint(s) HÀ ? Value Units
Chapter11: Properties Of Solutions
Section: Chapter Questions
Problem 5RQ: Define the terms in Raoults law. Figure 10-9 illustrates the net transfer of water molecules from...
Related questions
Question
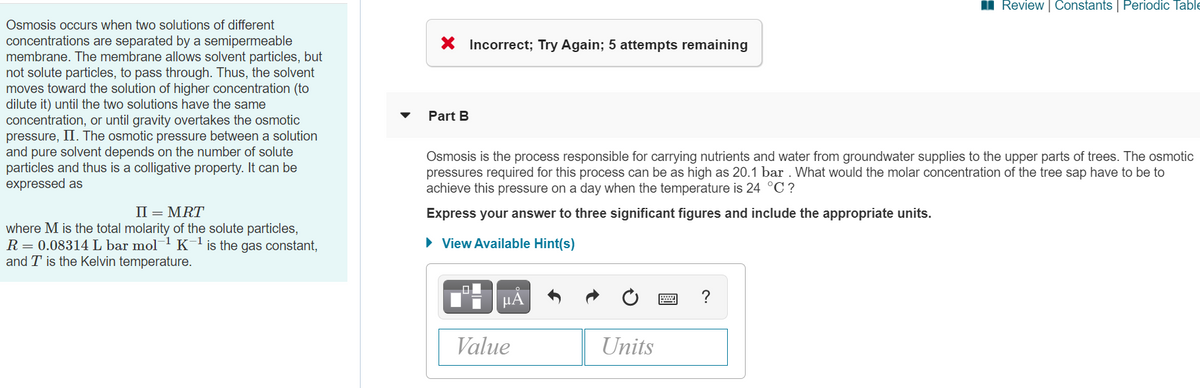
Transcribed Image Text:I Review | Constants | Periodic Table
Osmosis occurs when two solutions of different
concentrations are separated by a semipermeable
membrane. The membrane allows solvent particles, but
not solute particles, to pass through. Thus, the solvent
moves toward the solution of higher concentration (to
dilute it) until the two solutions have the same
concentration, or until gravity overtakes the osmotic
pressure, II. The osmotic pressure between a solution
and pure solvent depends on the number of solute
particles and thus is a colligative property. It can be
expressed as
X Incorrect; Try Again; 5 attempts remaining
Part B
Osmosis is the process responsible for carrying nutrients and water from groundwater supplies to the upper parts of trees. The osmotic
pressures required for this process can be as high as 20.1 bar . What would the molar concentration of the tree sap have to be to
achieve this pressure on a day when the temperature is 24 °C ?
II = MRT
Express your answer to three significant figures and include the appropriate units.
where M is the total molarity of the solute particles,
R= 0.08314L bar mol K'is the gas constant,
and T is the Kelvin temperature.
• View Available Hint(s)
HA
?
Value
Units
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

Chemistry: Matter and Change
Chemistry
ISBN:
9780078746376
Author:
Dinah Zike, Laurel Dingrando, Nicholas Hainen, Cheryl Wistrom
Publisher:
Glencoe/McGraw-Hill School Pub Co

Chemical Principles in the Laboratory
Chemistry
ISBN:
9781305264434
Author:
Emil Slowinski, Wayne C. Wolsey, Robert Rossi
Publisher:
Brooks Cole