Paper spacer ВATTERY Zn2 (aq)2e Moist paste of ZnCl2 and NH4CI Layer of MnO2 Zn(s) Anode: Cathode 2NH (aq)2MnO2(s) + 2e" -= Mn2O3(s) 2NH3(aq) +H2O(I) Zn2 (aq) +2NH3(aq) H20(I)Mn2O3(s) Graphite cathode Overall: Zn(s) + 2NH (aq) + 2MnO2(s) Zine anode Figure 19.7 Interior section of a dry cell of the kind used in flashlights and transistor radios. Actually, the cell is not completely dry, as it contains a moist electrolyte paste. Cathode (steel) Zn(Hg)20H (aq) ZnO(s) H2O(l) + 2e Cathode: HgO(s) + H2O(l) + 2e >Hg() +20H (aq) Zn(Hg)HgO(s) ZnO(s) + Hg(/) Anode (Zn can) Insulation Anode: Overall: Removable cap Anode Cathode Electrolyte solution containing KOH and paste of Zn(OH)2 and HgO Figure 19.8 Interior section of a mercury battery HSO, electrolyte 3 PbSO4(s)2e Pb(s) SO(aq) PbSO&(s) 2H2O(I) Negative plates (lead grills filled with spongy lead) -> PbO2(s) 4H* (aq) + SO% (aq) + 2e Pb(s)PbO2(s) + 4H*(aq) 2SO (aq) Positive plates (lead grills filled with PhO) Overall: 2PBSO4(s) + 2H2O(/)
Paper spacer ВATTERY Zn2 (aq)2e Moist paste of ZnCl2 and NH4CI Layer of MnO2 Zn(s) Anode: Cathode 2NH (aq)2MnO2(s) + 2e" -= Mn2O3(s) 2NH3(aq) +H2O(I) Zn2 (aq) +2NH3(aq) H20(I)Mn2O3(s) Graphite cathode Overall: Zn(s) + 2NH (aq) + 2MnO2(s) Zine anode Figure 19.7 Interior section of a dry cell of the kind used in flashlights and transistor radios. Actually, the cell is not completely dry, as it contains a moist electrolyte paste. Cathode (steel) Zn(Hg)20H (aq) ZnO(s) H2O(l) + 2e Cathode: HgO(s) + H2O(l) + 2e >Hg() +20H (aq) Zn(Hg)HgO(s) ZnO(s) + Hg(/) Anode (Zn can) Insulation Anode: Overall: Removable cap Anode Cathode Electrolyte solution containing KOH and paste of Zn(OH)2 and HgO Figure 19.8 Interior section of a mercury battery HSO, electrolyte 3 PbSO4(s)2e Pb(s) SO(aq) PbSO&(s) 2H2O(I) Negative plates (lead grills filled with spongy lead) -> PbO2(s) 4H* (aq) + SO% (aq) + 2e Pb(s)PbO2(s) + 4H*(aq) 2SO (aq) Positive plates (lead grills filled with PhO) Overall: 2PBSO4(s) + 2H2O(/)
Principles of Instrumental Analysis
7th Edition
ISBN:9781305577213
Author:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Chapter24: Coulometry
Section: Chapter Questions
Problem 24.4QAP: Halide ions can he deposited at a silver anode, the reaction being Ag(s) + X- AgX(s) +e- Suppose...
Related questions
Question
Calculate the emf cell of each overall reactions. Then, compare them with the actual emf cell of a dry cell, a mercury battery, and also accu!
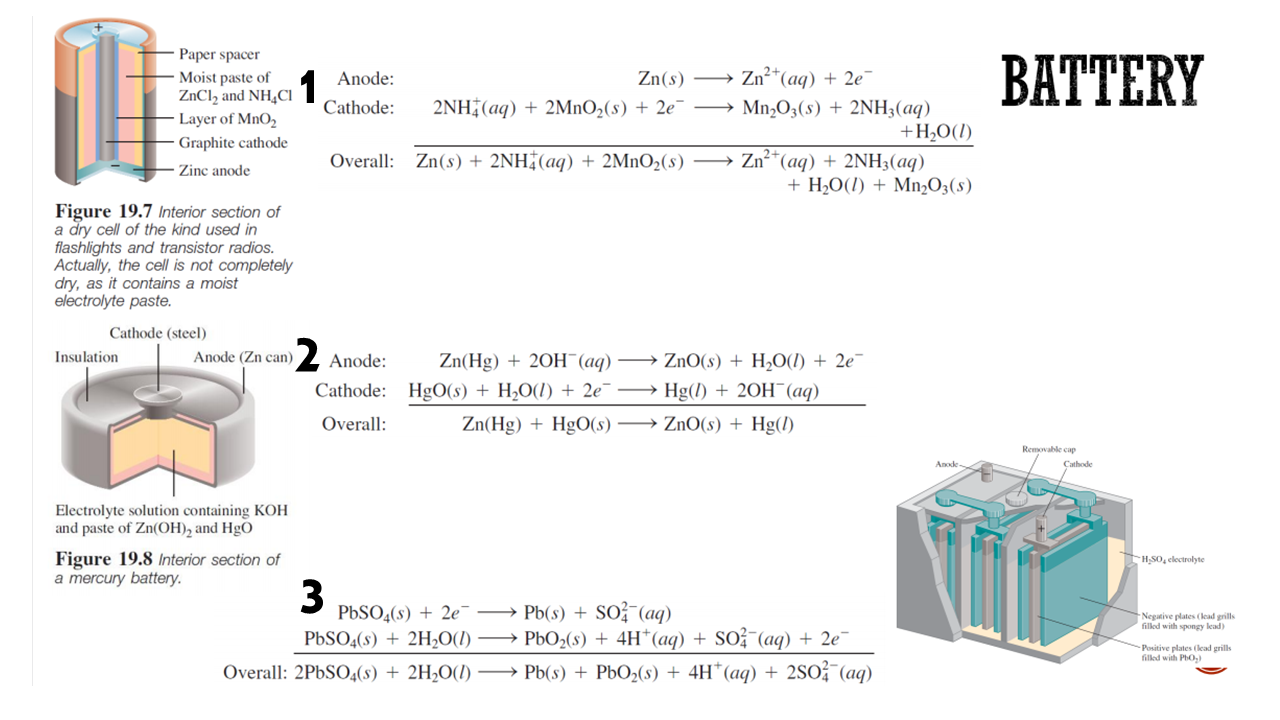
Transcribed Image Text:Paper spacer
ВATTERY
Zn2 (aq)2e
Moist paste of
ZnCl2 and NH4CI
Layer of MnO2
Zn(s)
Anode:
Cathode
2NH (aq)2MnO2(s) + 2e" -= Mn2O3(s) 2NH3(aq)
+H2O(I)
Zn2 (aq) +2NH3(aq)
H20(I)Mn2O3(s)
Graphite cathode
Overall: Zn(s) + 2NH (aq) + 2MnO2(s)
Zine anode
Figure 19.7 Interior section of
a dry cell of the kind used in
flashlights and transistor radios.
Actually, the cell is not completely
dry, as it contains a moist
electrolyte paste.
Cathode (steel)
Zn(Hg)20H (aq) ZnO(s) H2O(l) + 2e
Cathode: HgO(s) + H2O(l) + 2e >Hg() +20H (aq)
Zn(Hg)HgO(s) ZnO(s) + Hg(/)
Anode (Zn can)
Insulation
Anode:
Overall:
Removable cap
Anode
Cathode
Electrolyte solution containing KOH
and paste of Zn(OH)2 and HgO
Figure 19.8 Interior section of
a mercury battery
HSO, electrolyte
3
PbSO4(s)2e Pb(s) SO(aq)
PbSO&(s) 2H2O(I)
Negative plates (lead grills
filled with spongy lead)
-> PbO2(s) 4H* (aq) + SO% (aq) + 2e
Pb(s)PbO2(s) + 4H*(aq) 2SO (aq)
Positive plates (lead grills
filled with PhO)
Overall: 2PBSO4(s) + 2H2O(/)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 7 steps with 7 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning