PART 2: DRAWING MOLECULES TO SHOW 3-DIMENSIONALITY MODEL 2: Line, Wedge and Dash Drawings Line: In the plane of the paper: Wedge: Coming forward, in front of the plane of the paper: Dash: Going backward, behind the plane of the paper: * 8. Where is each of the 5 atoms in the molecule CHECIB"? In the plane of the paper In front of the plane of the paper . Behind the plane of the paper- H- Br 9. Using the Model screen, add bonding units (•) to the central atom (0). Using lines, wedges and dashes from Model 2, draw each molecule's shape. Bonding Electron Units Around Central Atom Drawing of Shape Electron Geometry Bond Angles 2 Linear 180° trigonal planar 3 120°
PART 2: DRAWING MOLECULES TO SHOW 3-DIMENSIONALITY MODEL 2: Line, Wedge and Dash Drawings Line: In the plane of the paper: Wedge: Coming forward, in front of the plane of the paper: Dash: Going backward, behind the plane of the paper: * 8. Where is each of the 5 atoms in the molecule CHECIB"? In the plane of the paper In front of the plane of the paper . Behind the plane of the paper- H- Br 9. Using the Model screen, add bonding units (•) to the central atom (0). Using lines, wedges and dashes from Model 2, draw each molecule's shape. Bonding Electron Units Around Central Atom Drawing of Shape Electron Geometry Bond Angles 2 Linear 180° trigonal planar 3 120°
Chemistry
10th Edition
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Chapter10: Liquids And Solids
Section: Chapter Questions
Problem 113AE: Some of the physical properties of H2O and D2O are as follows: Property H2O D2O Density at 20C...
Related questions
Question
100%
Transcribed Image Text:AutoSave
VSEPR-Handout -
P Search
ff
Jacobson, Ariel
JA
File
Home
Insert
Draw
Design
Layout
References
Mailings
Review
View
Help
EndNote X9
A Share
P Comments
X Cut
Find
Cambria (Body) v 12 - A A Aav A
• AaBbC AaBbCcl 1. AaBbC AaBbCcD AaBbCcD AaBbCcD
LB Copy
O Replace
Paste
BIU v ab x, x A - erAv
I PHET Bu... 1 PHET He... 1 PHET Nu... 1 PHET Ta... 1 PHET Ta... 1 PHET Te...
Dictate
Sensitivity
Editor
Insert
S Format Painter
A Select v
Symbols
Clipboard
Font
Paragraph
Styles
Editing
Voice
Sensitivity
Editor
LessonPix
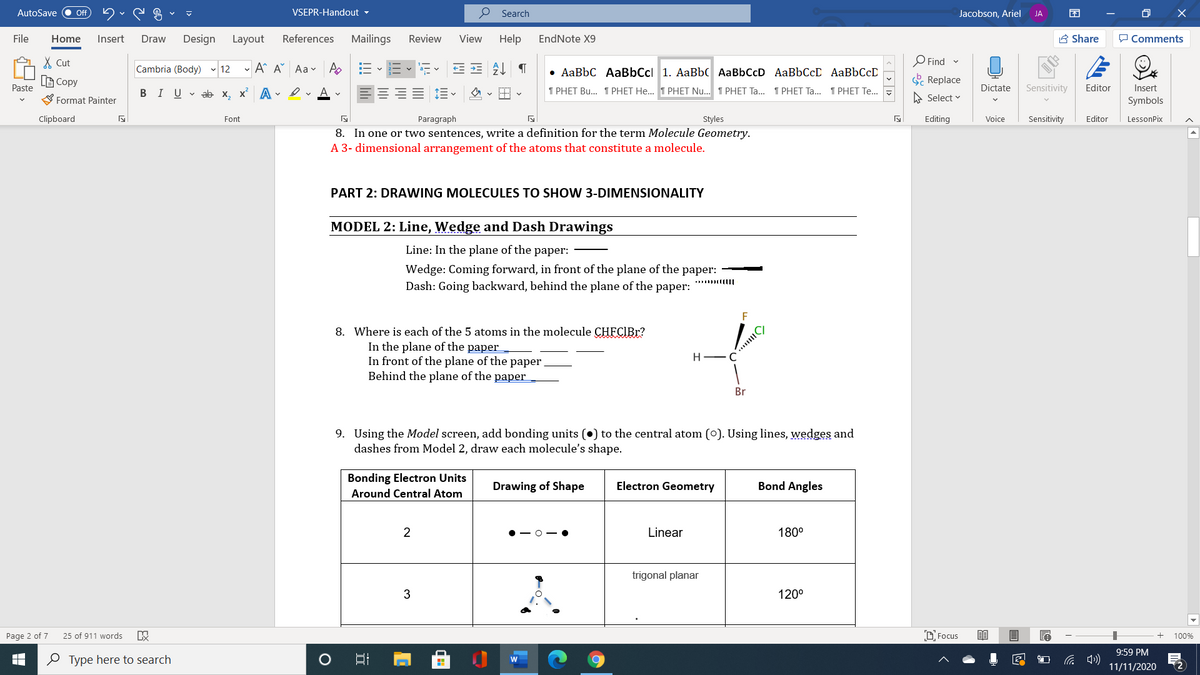
8. In one or two sentences, write a definition for the term Molecule Geometry.
A 3- dimensional arrangement of the atoms that constitute a molecule.
PART 2: DRAWING MOLECULES TO SHOW 3-DIMENSIONALITY
MODEL 2: Line, Wedge and Dash Drawings
Line: In the plane of the paper:
Wedge: Coming forward, in front of the plane of the paper:
Dash: Going backward, behind the plane of the paper:
8. Where is each of the 5 atoms in the molecule CHFCIBR?
In the plane of the paper
In front of the plane of the paper
Behind the plane of the paper
H.
Br
9. Using the Model screen, add bonding units (•) to the central atom (0). Using lines, wedges and
dashes from Model 2, draw each molecule's shape.
Bonding Electron Units
Drawing of Shape
Electron Geometry
Bond Angles
Around Central Atom
2
Linear
180°
trigonal planar
120°
Page 2 of 7
D Focus
25 of 911 words
100%
9:59 PM
O Type here to search
11/11/2020
<> I>
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
