I Review | Constants | Periodic Table wei гgy of The energy difference between two energy shells The energy difference between two energy shels is calculated using the formula aree AE- E where Er initial shell is the fourth shell, and the final shell is the first shell. Thus, the change in energy bolwoen the fourth and the tirst shell is denoted as AE-E-E The energy corresponding to a shell is calculated using is the energy of the final shell and E is the eneray of the nitial sholl fron which the transition occurs For oxample, for the transition n-4 to n -1, the E,--2.179 x 10o-/(2) J where a is the shell number Part B- Calculate the energy difference Calculate the energy difference for a transition in the Paschen series for a transition from the highit energy shell n 4 Express your answer to four significant figures and include the appropriate units. way of to the • View Available Hint(s) ies ? Value Units Submit Previous Answers
I Review | Constants | Periodic Table wei гgy of The energy difference between two energy shells The energy difference between two energy shels is calculated using the formula aree AE- E where Er initial shell is the fourth shell, and the final shell is the first shell. Thus, the change in energy bolwoen the fourth and the tirst shell is denoted as AE-E-E The energy corresponding to a shell is calculated using is the energy of the final shell and E is the eneray of the nitial sholl fron which the transition occurs For oxample, for the transition n-4 to n -1, the E,--2.179 x 10o-/(2) J where a is the shell number Part B- Calculate the energy difference Calculate the energy difference for a transition in the Paschen series for a transition from the highit energy shell n 4 Express your answer to four significant figures and include the appropriate units. way of to the • View Available Hint(s) ies ? Value Units Submit Previous Answers
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter4: Introduction To Quantum Mechanics
Section: Chapter Questions
Problem 38P: Using a simple particle-in-a-box model for the multiple bonding in 1,2-butadiene (see Example 4.7)...
Related questions
Question
Transcribed Image Text:I Review | Constants | Periodic Table
wei
rgy of
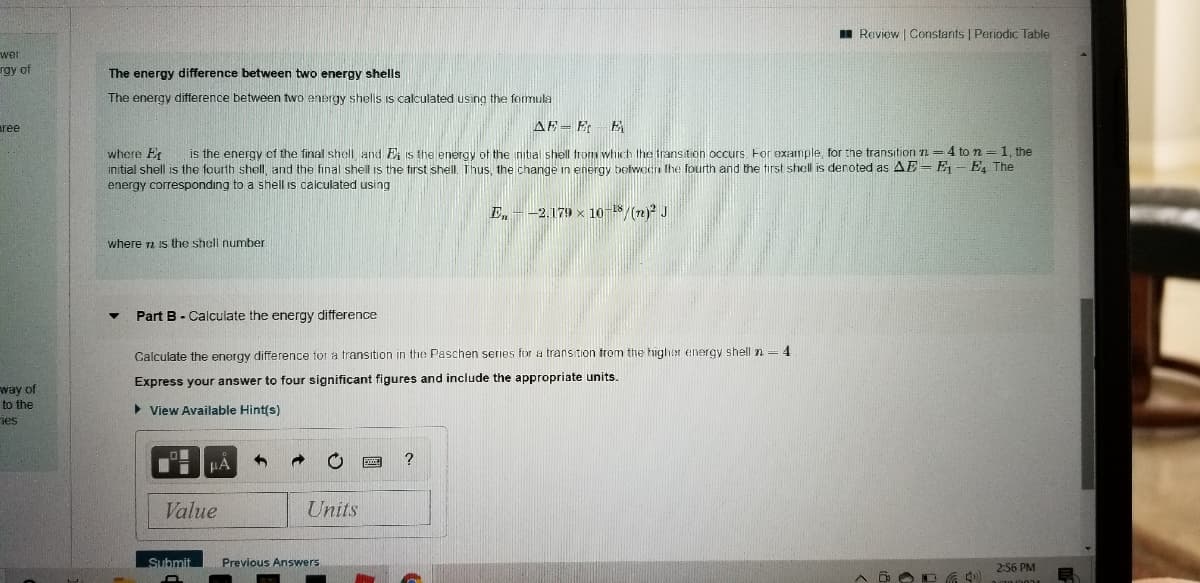
The energy difference between two energy shells
The energy difference between two energy shells Is calculated using the formula
ree
AE- E- E
is the energy of the final shell and E, is the energy of the nitial shell fromm which the transition occurs. For example, for the transition n = 4 to n =1, the
where Er
initial shell is the fourth shell, and the final shell is the first shell. Thus, the change in energy between the fourth and the tirst shell is deroted as AE= E – E4 The
energy corresponding to a shell is calculated using
En
-2.179 x 10 /(2)? J
where a is the shell number
Part B - Calculate the energy difference
Calculate the energy difference for a transition in the Paschen series for a transition trom the higher energy shell n = 4
Express your answer to four significant figures and include the appropriate units.
way of
to the
• View Available Hint(s)
ies
?
HA
Value
Units
Submit
Previous Answers
2:56 PM
Transcribed Image Text:CHM 1045
michael
<Post Lecture Homework Chapter 07
Interactive Activity-Energy Levels of a Hydrogen Atom
< 7 of 9 >
I Review | Constants | Periodic Table
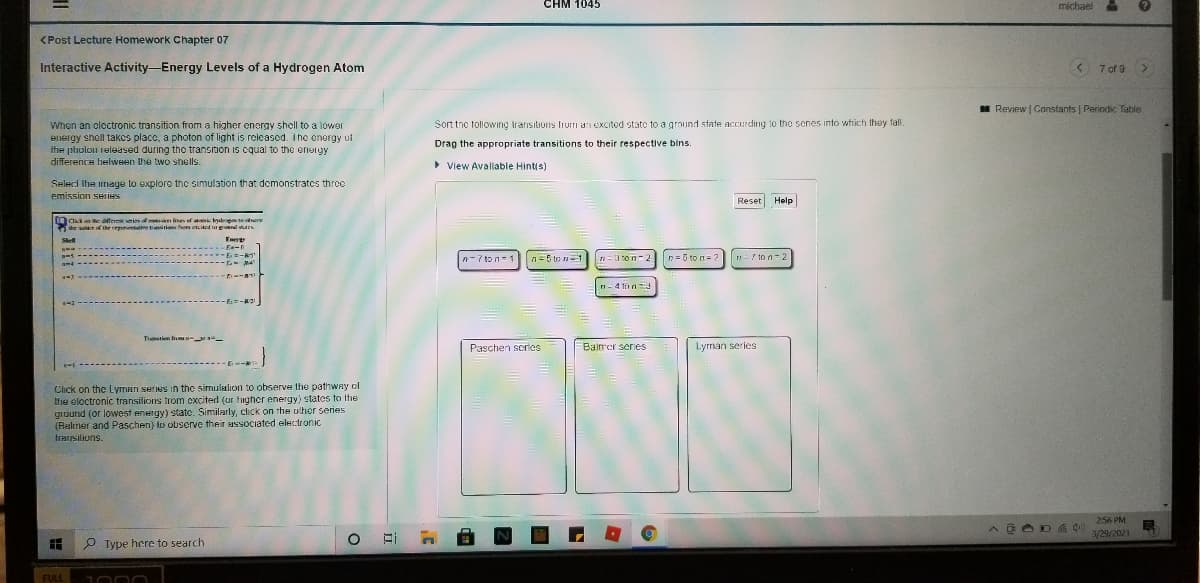
When an oloctronic transition from a higher energy sholl to a lower
energy shell takes placc, a photon of light is released. The energy of
the pholou released during tho transition is cqual to the eneigy
difference belween the two shells.
Sort tnc following transitions liurri an excited state to a ground state accurding 10 the senes into which they fall.
Drag the appropriate transitions to their respective bins.
• View Avallable Hintis)
Seleci Ihe image to explore thc simulation that demonstrates three
emission SHIIHS
Reset Help
Ckt an he differeies n lines ef ak lydropmte e
de unr of the rpenve tiriens fo encitd a gonl vars
Energ
-Ea-n
- 8s
Sel
E =-
-- M4
n = 5 to n= 2
n-7 ton-1 n=5 to n
n=3 ton-2
ロニ/pn=2
ri- 4 tnn
-E:=-R2
Ttien from --
Paschen scrles
Balrer series
Lyrman series
Click on the Lyman series in the simulalion to observe the pathway ol
thhe eloctronic transilions trom exciterd (ur fugher energy) states to the
ground (or lowest enengy) state. Similarly, click on the ulher series
(Realiner and Paschen) to observe their ussociated electronic
trarisilions.
256 PM
3/29/2021
P Type here to search
FULL
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning