Part A: Determination of Densities of Pure Water and Calcium Chloride Solutions (1) Temperature of water (to nearest 0.5 °C) 22.5 °C (2) Mass of empty volumetric flask with stopper 20.3872 g (3) Mass of volumetric flask, stopper, and water 21.3762 d (4) Mass of water in the volumetric flask (5) Calculated density of water at temperature (4) / 1.00 mL) - g/mL
Part A: Determination of Densities of Pure Water and Calcium Chloride Solutions (1) Temperature of water (to nearest 0.5 °C) 22.5 °C (2) Mass of empty volumetric flask with stopper 20.3872 g (3) Mass of volumetric flask, stopper, and water 21.3762 d (4) Mass of water in the volumetric flask (5) Calculated density of water at temperature (4) / 1.00 mL) - g/mL
Chapter7: Statistical Data Treatment And Evaluation
Section: Chapter Questions
Problem 7.21QAP
Related questions
Question
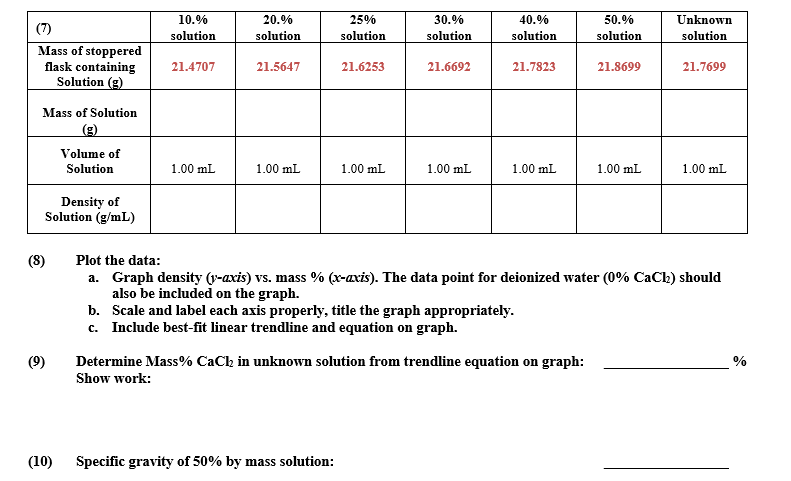
Transcribed Image Text:10.%
20.%
25%
30.%
40.%
50.%
Unknown
(7)
solution
solution
solution
solution
solution
solution
solution
Mass of stoppered
flask containing
Solution (g)
21.4707
21.5647
21.6253
21.6692
21.7823
21.8699
21.7699
Mass of Solution
(3)
Volume of
Solution
1.00 mL
1.00 mL
1.00 mL
1.00 mL
1.00 mL
1.00 mL
1.00 mL.
Density of
Solution (g/mL)
(8)
Plot the data:
a. Graph density (r-axis) vs. mass % (x-avis). The data point for deionized water (0% CaClh) should
also be included on the graph.
b. Scale and label each axis properly, title the graph appropriately.
c. Include best-fit linear trendline and equation on graph.
Determine Mass% CaCh in unknown solution from trendline equation on graph:
Show work:
(10)
Specific gravity of 50% by mass solution:
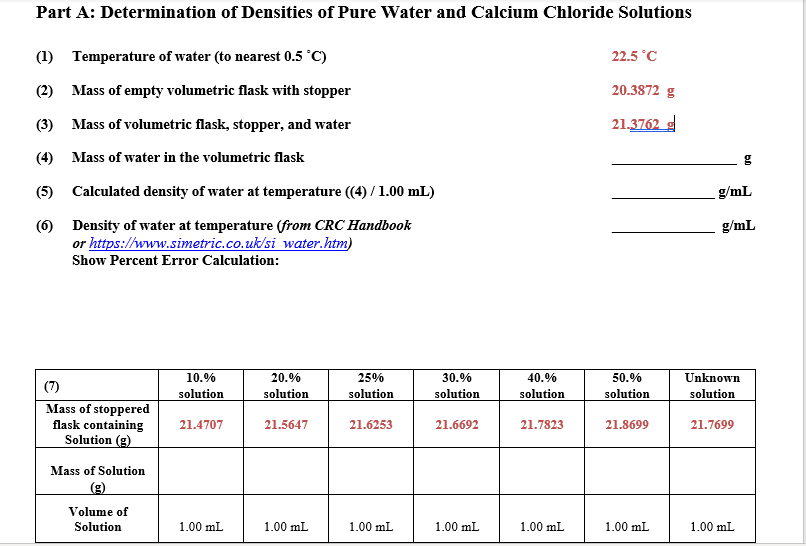
Transcribed Image Text:Part A: Determination of Densities of Pure Water and Calcium Chloride Solutions
(1) Temperature of water (to nearest 0.5 °C)
22.5 °C
(2) Mass of empty volumetric flask with stopper
20.3872 g
(3) Mass of volumetric flask, stopper, and water
21.3762 g
(4) Mass of water in the volumetric flask
g
(5) Calculated density of water at temperature (4) / 1.00 mL)
g/mL
(6) Density of water at temperature (from CRC Handbook
or https://www.simetric.co.uk/si water.htm)
g/mL
Show Percent Error Calculation:
10.%
20.%
25%
30.%
40.%
50.%
Unknown
(7)
solution
solution
solution
solution
solution
solution
solution
Mass of stoppered
flask containing
Solution (g)
21.4707
21.5647
21.6253
21.6692
21.7823
21.8699
21.7699
Mass of Solution
Volume of
Solution
1.00 mL
1.00 mL
1.00 mL
1.00 mL
1.00 mL
1.00 mL
1.00 mL
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

