Molarity of NaOH (phenophthalein) 0.1132 Volume of HC2H3O2 (acetic acid) 25.00 ml. vinegar %3D TRIAL A C Initial Volume (ml.) 0.15 0.43 0.31 Final Volume (ml.) 35.81 34.02 33.96 CULATIONS: TRIAL A. C Volume NaOH used Moles NaOH MHC2 H3O2 Rejected Molarities: Arithmetic Mean: II
Molarity of NaOH (phenophthalein) 0.1132 Volume of HC2H3O2 (acetic acid) 25.00 ml. vinegar %3D TRIAL A C Initial Volume (ml.) 0.15 0.43 0.31 Final Volume (ml.) 35.81 34.02 33.96 CULATIONS: TRIAL A. C Volume NaOH used Moles NaOH MHC2 H3O2 Rejected Molarities: Arithmetic Mean: II
Chapter3: Statistical Tests With Excel
Section: Chapter Questions
Problem 6P
Related questions
Question
100%
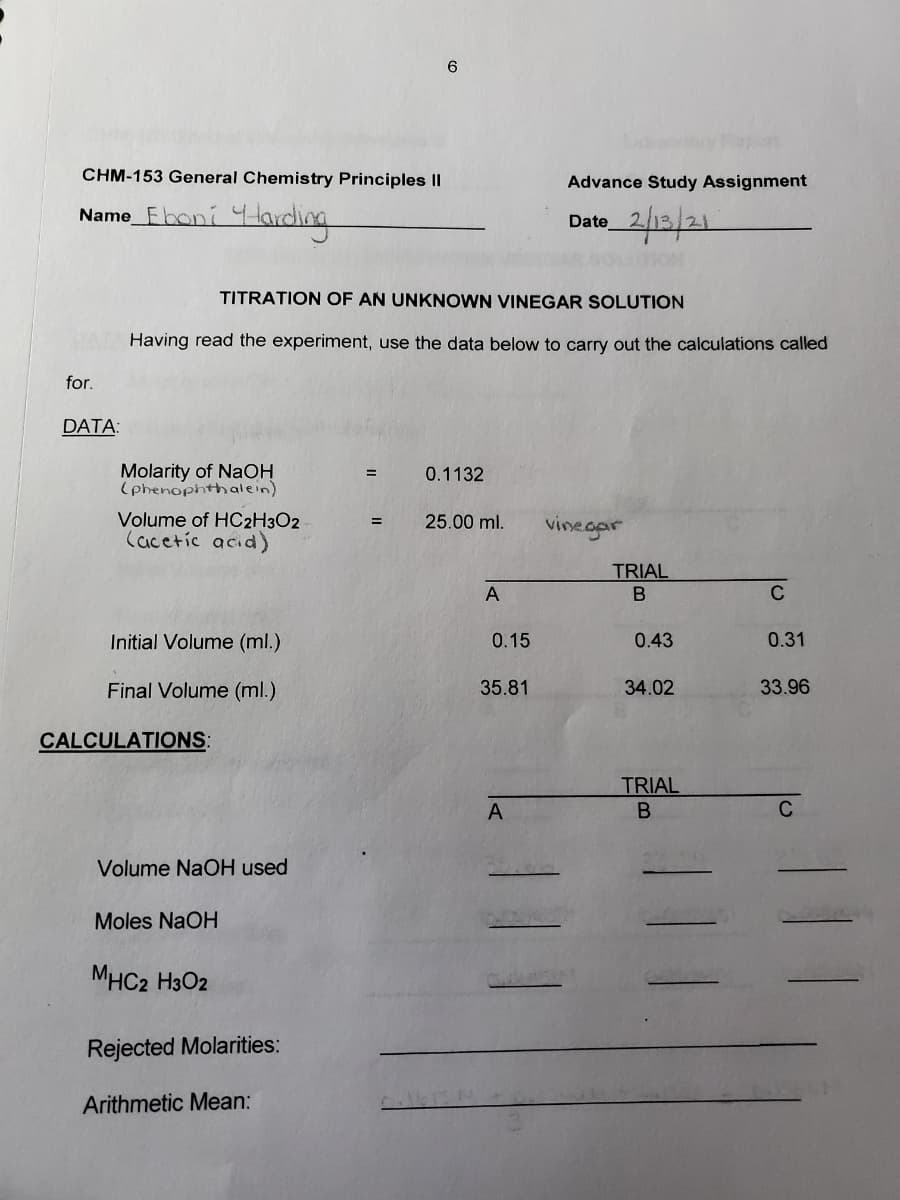
Transcribed Image Text:CHM-153 General Chemistry Principles ||
Advance Study Assignment
Name_Fboni 4larding
2/12/21
Date
TITRATION OF AN UNKNOWN VINEGAR SOLUTION
Having read the experiment, use the data below to carry out the calculations called
for.
DATA:
Molarity of NaOH
(phenophthalein)
0.1132
Volume of HC2H3O2
Cacetic acid)
25.00 ml.
vinegar
=
TRIAL
A
C
Initial Volume (ml.)
0.15
0.43
0.31
Final Volume (ml.)
35.81
34.02
33.96
CALCULATIONS:
TRIAL
C
Volume NaOH used
Moles NaOH
MHC2 H3O2
Rejected Molarities:
Arithmetic Mean:
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning