Part A For each set of elements represented in this periodic table outline, identify the principal quantum number, n, and the azimuthal quantum number, £, for the highest energy electrons in an atom of one of those elements. Drag each label to the appropriate target. • View Available Hint(s) Reset Help 3 00 Part B The black line between elements 56 and 72 in the periodic table shown indicates that in the Lanthanide series elements 57 through 71 are listed below the main table, while in the Actinide series elements 89-103 are listed below the main table. Elements 72 and 104 are listed in main table. Identify the outer electron configuration of each element shown in this periodic table outline. Drag each label to the appropriate target. > View Available Hint(s) Reset Help 7s 5f146d? 7s 5f14 7s 5f146d107p% 58 4d10 7s 6d² 5s23f144d® 5824d105p® 7s 6d05f14 5s' 3s 3p? 6s! 7s27d? 7827f14 7s27d107p 3s 2p 5825d 6s?
Part A For each set of elements represented in this periodic table outline, identify the principal quantum number, n, and the azimuthal quantum number, £, for the highest energy electrons in an atom of one of those elements. Drag each label to the appropriate target. • View Available Hint(s) Reset Help 3 00 Part B The black line between elements 56 and 72 in the periodic table shown indicates that in the Lanthanide series elements 57 through 71 are listed below the main table, while in the Actinide series elements 89-103 are listed below the main table. Elements 72 and 104 are listed in main table. Identify the outer electron configuration of each element shown in this periodic table outline. Drag each label to the appropriate target. > View Available Hint(s) Reset Help 7s 5f146d? 7s 5f14 7s 5f146d107p% 58 4d10 7s 6d² 5s23f144d® 5824d105p® 7s 6d05f14 5s' 3s 3p? 6s! 7s27d? 7827f14 7s27d107p 3s 2p 5825d 6s?
Chemistry: Principles and Practice
3rd Edition
ISBN:9780534420123
Author:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Chapter7: Electronic Structure
Section: Chapter Questions
Problem 7.102QE
Related questions
Question
The periodic table lists all known elements arranged by atomic number . Atomic number is the nuclear charge, the number of protons in the nucleus of an an atom of a particular element. For a neutral atom, the number of protons is equal to the number of electrons. Each column of the table, called a group, contains elements with the same number of valence electrons that are in different quantum levels. Each row of the table, called a period, contains elements with differing numbers of valence electrons that are in the same principal quantum level. The four main blocks of the table (s, p, d, and f) contain elements whose highest energy electrons have the same azimuthal quantum number (ℓ).
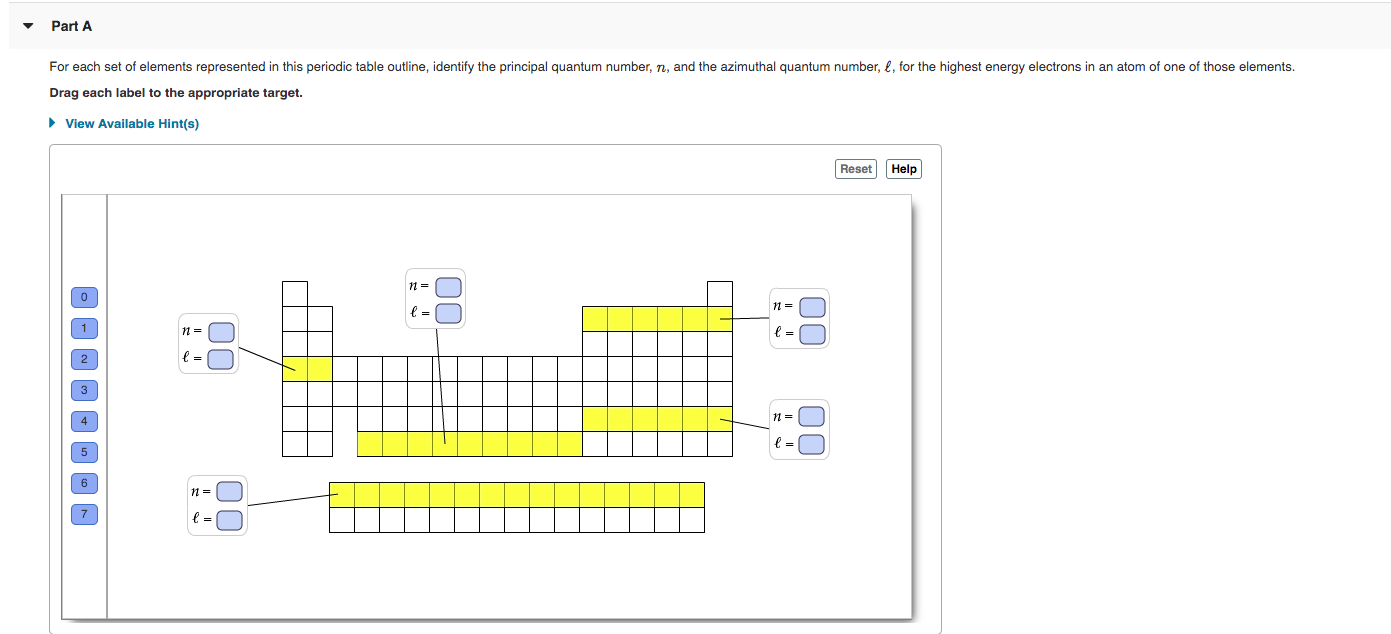
Transcribed Image Text:Part A
For each set of elements represented in this periodic table outline, identify the principal quantum number, n, and the azimuthal quantum number, £, for the highest energy electrons in an atom of one of those elements.
Drag each label to the appropriate target.
• View Available Hint(s)
Reset
Help
3
00
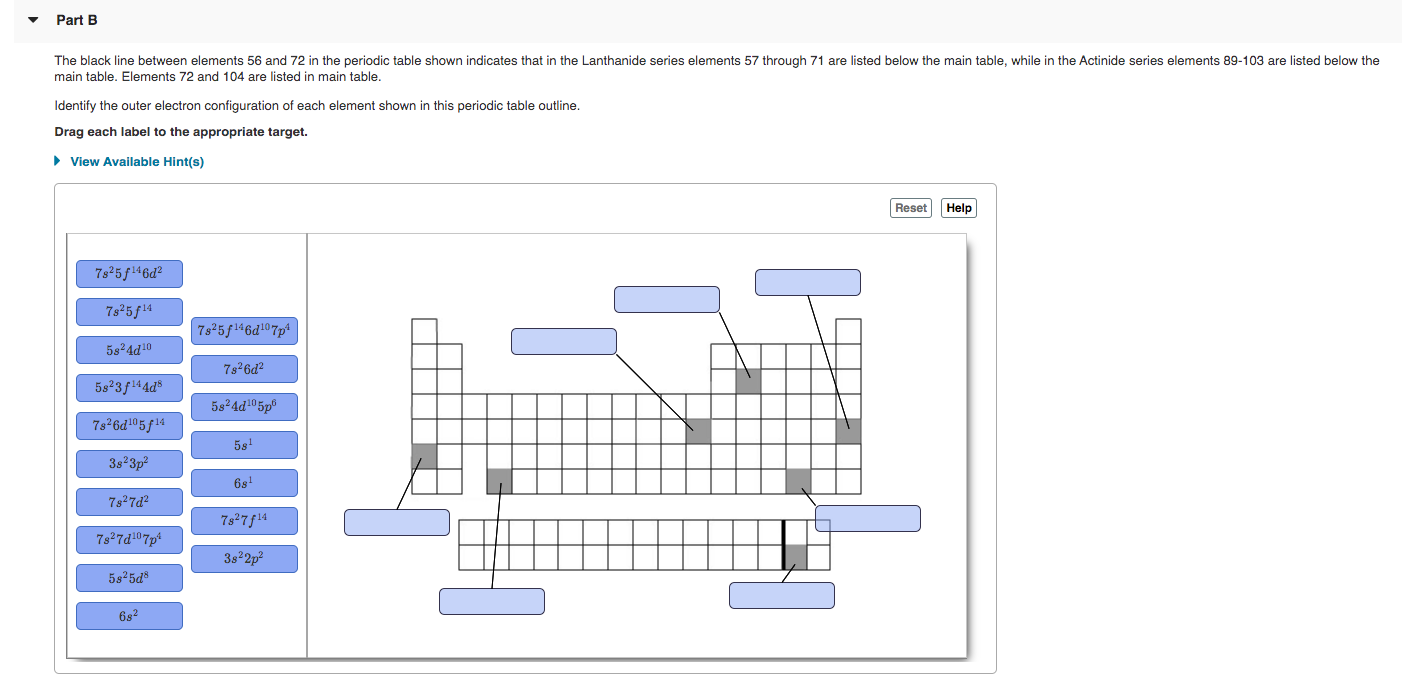
Transcribed Image Text:Part B
The black line between elements 56 and 72 in the periodic table shown indicates that in the Lanthanide series elements 57 through 71 are listed below the main table, while in the Actinide series elements 89-103 are listed below the
main table. Elements 72 and 104 are listed in main table.
Identify the outer electron configuration of each element shown in this periodic table outline.
Drag each label to the appropriate target.
> View Available Hint(s)
Reset
Help
7s 5f146d?
7s 5f14
7s 5f146d107p%
58 4d10
7s 6d²
5s23f144d®
5824d105p®
7s 6d05f14
5s'
3s 3p?
6s!
7s27d?
7827f14
7s27d107p
3s 2p
5825d
6s?
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning


Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning