Reset Help Previous Answer v Correct • Part B Complete an orbital dagram for scandium (Sc) Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. View Available Hints) Reset Helo 11 1 he s * Corect Part C cond n ot h Eledn conigations are a shothnd fu of an l degam des g wh coed r e for Le2 Ente the cample coiro a A Express your an complte oin e der of urbal ng en ng wout kspce bete orale For emle, le'esde dan V lle 1of1 Submal
Reset Help Previous Answer v Correct • Part B Complete an orbital dagram for scandium (Sc) Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at all. View Available Hints) Reset Helo 11 1 he s * Corect Part C cond n ot h Eledn conigations are a shothnd fu of an l degam des g wh coed r e for Le2 Ente the cample coiro a A Express your an complte oin e der of urbal ng en ng wout kspce bete orale For emle, le'esde dan V lle 1of1 Submal
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter11: Modern Atomic Theory
Section: Chapter Questions
Problem 126CP
Related questions
Question
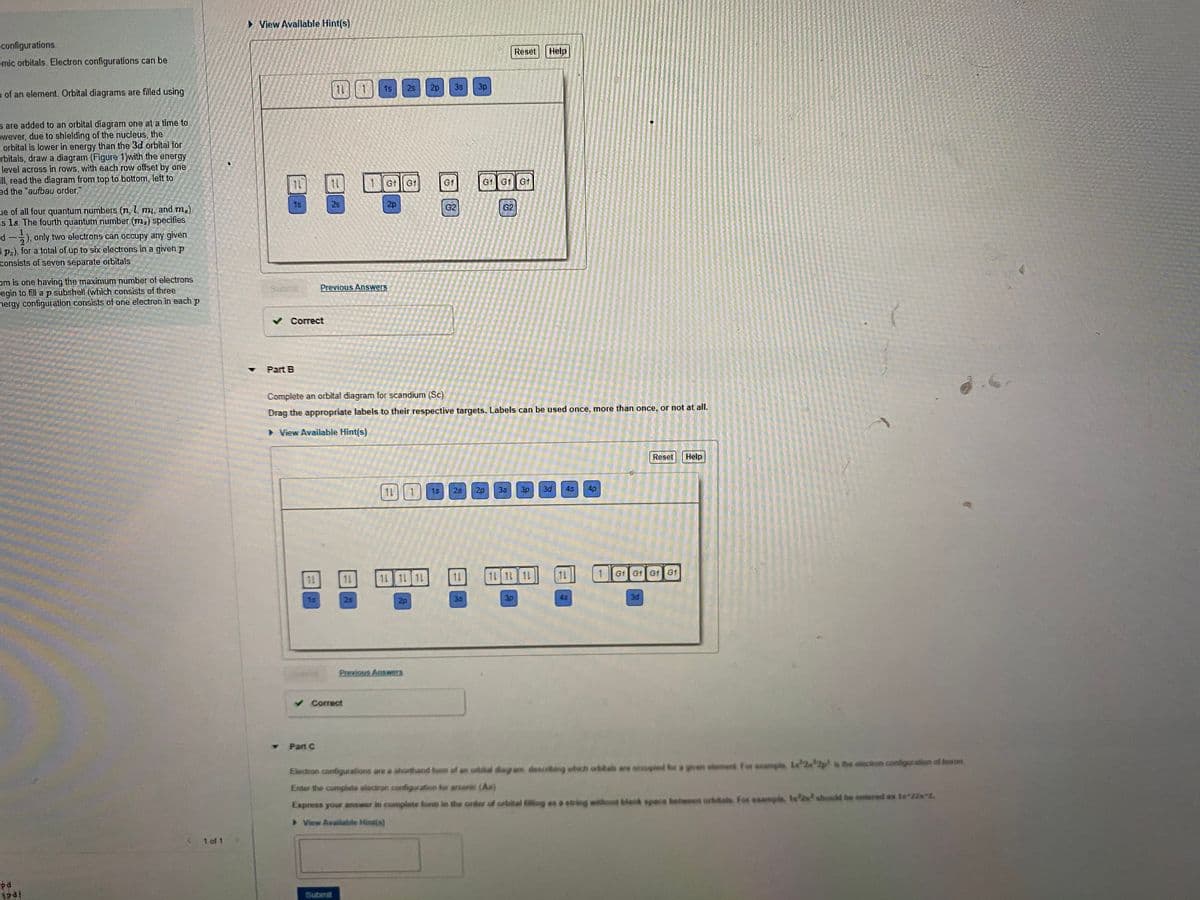
Transcribed Image Text:• View Available Hint(s)
configurations.
Reset Help
omic orbitals. Electron configurations can be
目日
11 1
1s
2s
2p
3s
3p
n of an element. Orbital diagrams are filled using
s are added to an orbital diagram one at a time to
owever, due to shielding of the nucleus, the
- orbital is lower in energy than the 3d orbital for
rbitals, draw a diagram (Figure 1)with the energy
level across in rows, with each row offset by one
Fll, read the diagram from top to bottom, left to
ed the "aufbau order."
1Gt G1
G1
G1 G1 G1
1s
28
2p
G2
G2
ue of all four quantum numbers (n, l, mi, and m).
is 1s. The fourth quantum number (m;) specifies
d
3 p:), for a total of up to six electrons in a given p
consists of seven separate orbitals.
), only two electrons can occupy any given
om is one having the maximum number of electrons
egin to fill a p subshell (which consists of three
nergy configuration consists of one electron in each p
Previous Answers
v Correct
Part B
Complete an orbital diagram for scandium (Sc)
Drag the appropriate labels to their respective targets. Labels can be used once, more than once, or not at alI.
> View Available Hint(s)
Reset
Help
11
25
20
3s
3p
3d
4s
4p
15
1L
11 1L 11
11
11 11 11
G1 G1 G1 G1
11
15
2s
2p
35
3p
45
3d
Previous Answers
v Correct
Part C
Electron configurations are a shorthand fom of an orbital diagram, descrting which orbita are accupied for a given sloment For ssample La 2 2p is the electron configuration of boron
Enter the complate electron configuration for arsenic (As)
Express your answer in complete lom in the order of orbital filling es a suing without blank space beteeen orbitals. For example, 1s should be entered as 1s 22s 2.
> View Available Hintis)
< 1 of 1
pa
Submit

Transcribed Image Text:Electron configurations are a shorthand form of an orbital diagram, describing which orbitals are occupied for a given element. For example, 1s 2s 2p is the electron configuration of boron.
Enter the complete electron configuration for arsenic (As).
Express your answer in complete form in the order of orbital filling as a string without blank space between orbitals. For example, 1s22s? should be entered as 1s^22s^2.
• View Available Hint(s)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning