Part B The actual measured mass of a potassium-39 atom is 38.963707 amu . What is the mass defect, Am, for a potassium-39 nucleus? Express your answer in atomic mass units using six significant figures. • View Available Hint(s) ? Am = 0.347837 amu Submit Previous Answers
Part B The actual measured mass of a potassium-39 atom is 38.963707 amu . What is the mass defect, Am, for a potassium-39 nucleus? Express your answer in atomic mass units using six significant figures. • View Available Hint(s) ? Am = 0.347837 amu Submit Previous Answers
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter9: Pre-quantum Mechanics
Section: Chapter Questions
Problem 9.18E
Related questions
Question
PLEASE HELP!!!!! just need part B and part D!!! WILL UPVOTE!!
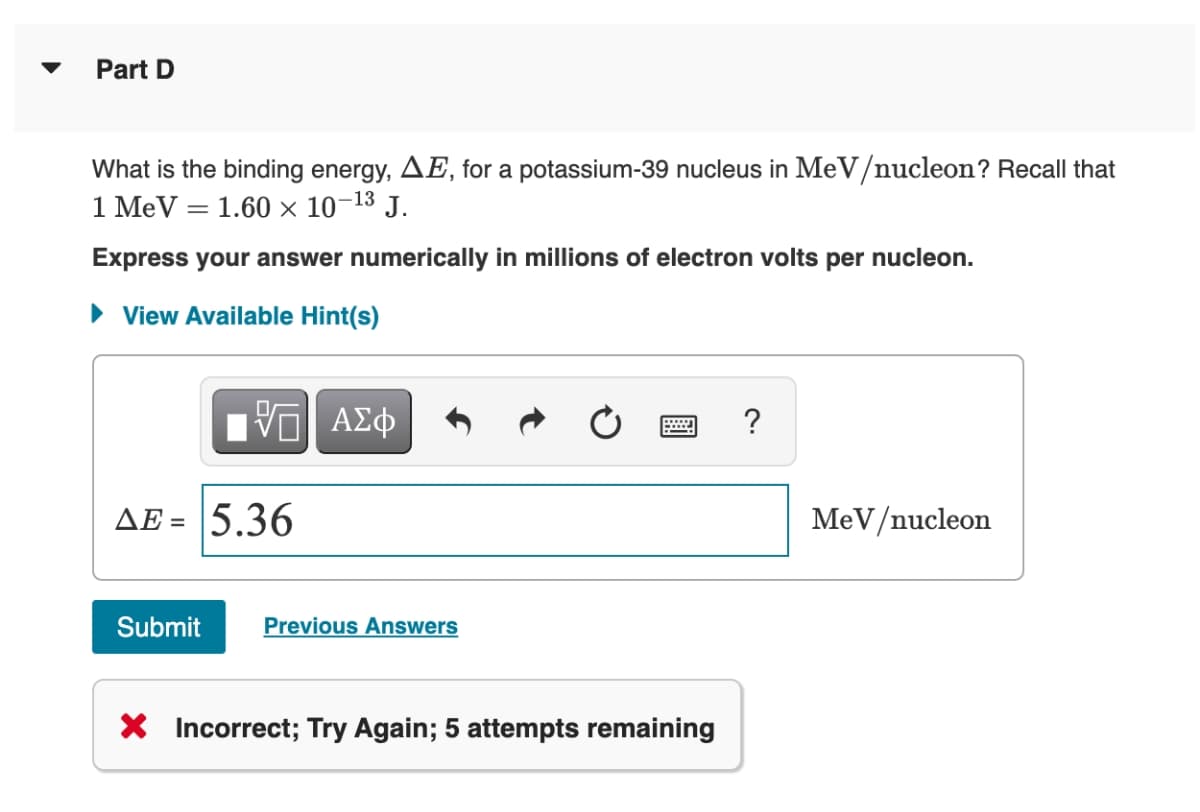
Transcribed Image Text:Part D
What is the binding energy, AE, for a potassium-39 nucleus in MeV/nucleon? Recall that
1 MeV = 1.60 ×x 10-13 J.
Express your answer numerically in millions of electron volts per nucleon.
• View Available Hint(s)
Πν ΑΣφ
?
AE = 5.36
MeV/nucleon
%3D
Submit
Previous Answers
X Incorrect; Try Again; 5 attempts remaining
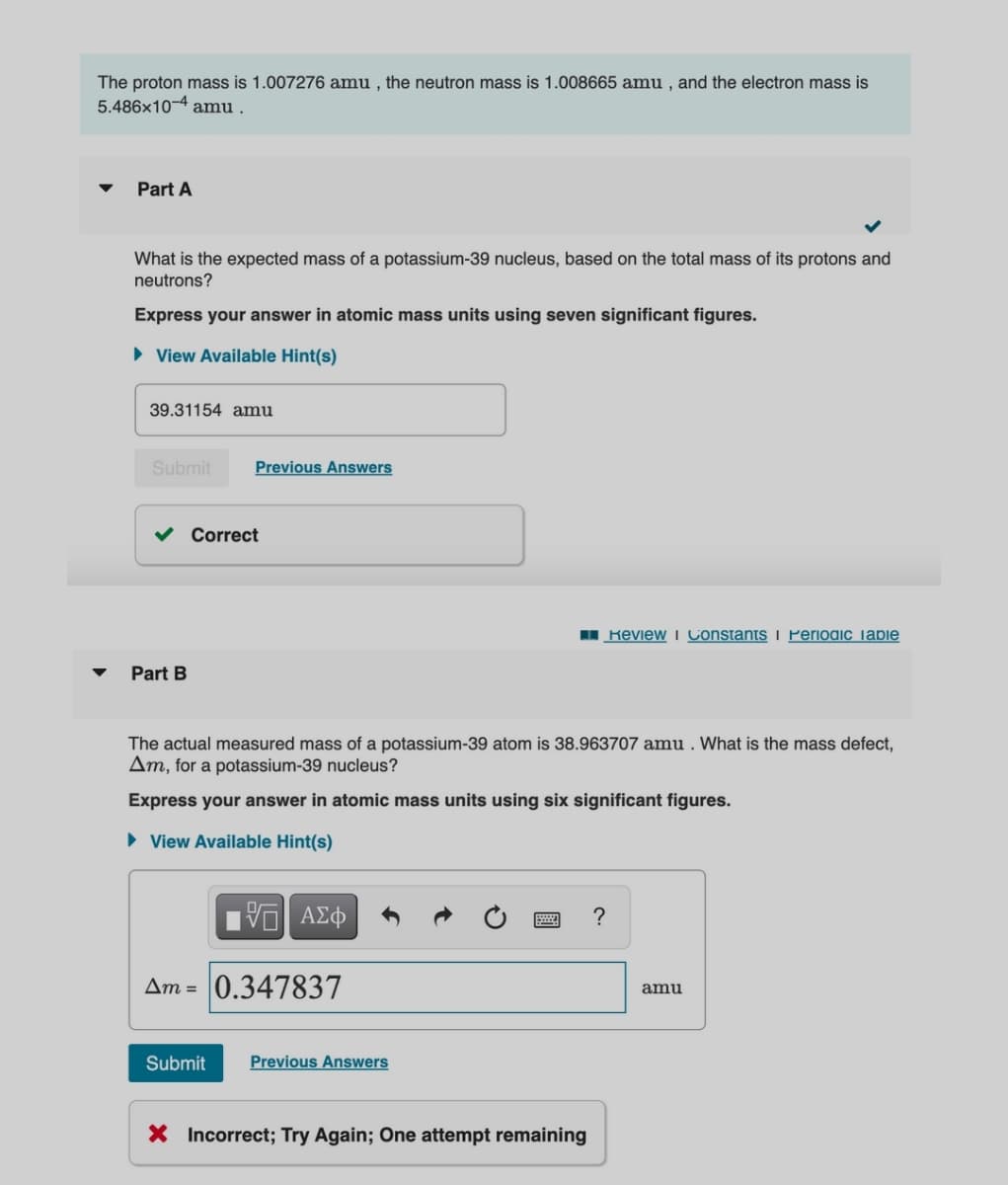
Transcribed Image Text:The proton mass is 1.007276 amu , the neutron mass is 1.008665 amu , and the electron mass is
5.486x10-4 amu .
Part A
What is the expected mass of a potassium-39 nucleus, based on the total mass of its protons and
neutrons?
Express your answer in atomic mass units using seven significant figures.
• View Available Hint(s)
39.31154 amu
Submit
Previous Answers
Correct
I Review i Constants i Periodic Tabie
Part B
The actual measured mass of a potassium-39 atom is 38.963707 amu . What is the mass defect,
Am, for a potassium-39 nucleus?
Express your answer in atomic mass units using six significant figures.
• View Available Hint(s)
?
Am = 0.347837
amu
Submit
Previous Answers
X Incorrect; Try Again; One attempt remaining
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning
