Part C If distilled water sells in a grocery store for $1.32 per gal what percentage of the sales price is represented by the cost of the energy? Express your answer using two significant figures.
Part C If distilled water sells in a grocery store for $1.32 per gal what percentage of the sales price is represented by the cost of the energy? Express your answer using two significant figures.
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter15: Gases,liquids, And Solids
Section: Chapter Questions
Problem 61E
Related questions
Question
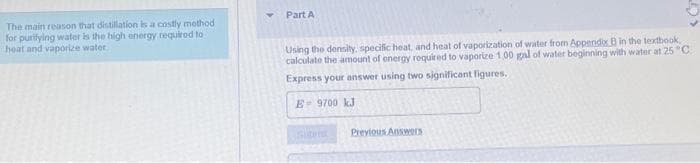
Transcribed Image Text:Part A
The main reason that distillation is a costly method
for purifying water is the high anergy requirod to
heat and vaporize water
Using the density, specific heat, and heat of vaporization of water from Appendix B in the textbook
calculate the amount of energy required to vaporize 1.00 gal of water beginning with water at 25 "C.
Express your answer using two significant ligures.
E 9700 kJ
Previous AnsweES
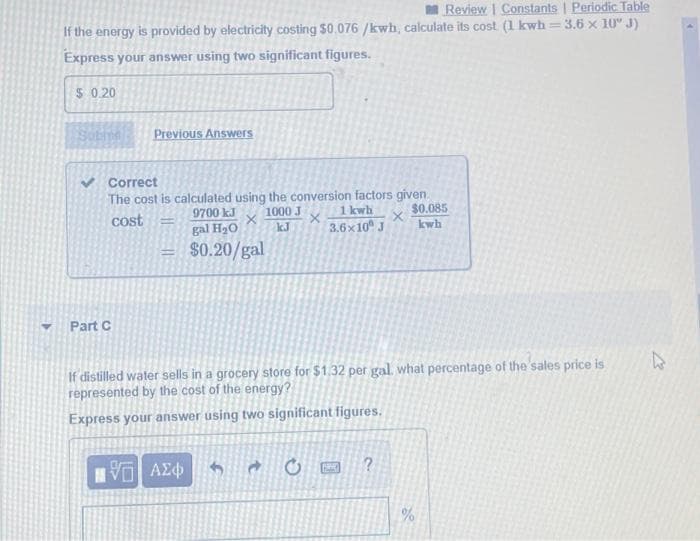
Transcribed Image Text:Review | Constants | Periodic Table
3.6 x 10" J)
If the energy is provided by electricity costing $0.076 /kwh, calculate its cost (1 kwh:
Express your answer using two significant figures.
$ 0.20
Previous Answers
Correct
The cost is calculated using the conversion factors given.
1000 J
kJ
$0.085
kwh
9700 kJ
1 kwh.
cost
gal H2O
3.6x10 J
$0.20/gal
Part C
If distilled water sells in a grocery store for $1.32 per gal. what percentage of the sales price is
represented by the cost of the energy?
Express your answer using two significant figures.
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry: Principles and Practice
Chemistry
ISBN:
9780534420123
Author:
Daniel L. Reger, Scott R. Goode, David W. Ball, Edward Mercer
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
