Part I: Line Bond Structures Instructions: After looking at the examples on page 2 and 3, complete the tables on each of the pages thereafter by drawing Line Bond Structures of the molecules given. When you are finished with all of the line bond structures, re-draw your structures on this first page and bring it to me to check. After I check your line bond structures, proceed to the Part II: Molecular Shape section. NH3 Br₂ H-N-H Br-Br Br-Br H H₂S Line Bond Structures and Molecular Geometry HCI H-Cl NI3 I-N-I 1 OF 2 F F H-N-H 14 c-c-c-c C H-CI: 00 .. :I-N-I: H 0: 2-methylbutane (C5H12) - see page 14 CC14 CI Cí (S₂ (-C) CS₂ (2)S 2X6e 12c - 20 S=C=S :: --Cr -G- .. ** O SO₂ ==0 :0-S=0 .. S = C = S 10e oc tet rule Acetone (C3H6O) - see page 15 C-C-C S, 6. 02 +2
Part I: Line Bond Structures Instructions: After looking at the examples on page 2 and 3, complete the tables on each of the pages thereafter by drawing Line Bond Structures of the molecules given. When you are finished with all of the line bond structures, re-draw your structures on this first page and bring it to me to check. After I check your line bond structures, proceed to the Part II: Molecular Shape section. NH3 Br₂ H-N-H Br-Br Br-Br H H₂S Line Bond Structures and Molecular Geometry HCI H-Cl NI3 I-N-I 1 OF 2 F F H-N-H 14 c-c-c-c C H-CI: 00 .. :I-N-I: H 0: 2-methylbutane (C5H12) - see page 14 CC14 CI Cí (S₂ (-C) CS₂ (2)S 2X6e 12c - 20 S=C=S :: --Cr -G- .. ** O SO₂ ==0 :0-S=0 .. S = C = S 10e oc tet rule Acetone (C3H6O) - see page 15 C-C-C S, 6. 02 +2
Introductory Chemistry: A Foundation
9th Edition
ISBN:9781337399425
Author:Steven S. Zumdahl, Donald J. DeCoste
Publisher:Steven S. Zumdahl, Donald J. DeCoste
Chapter12: Chemical Bonding
Section: Chapter Questions
Problem 37CR
Related questions
Question
Would you mind to check all the answer on this table because I try to understand the line bond structure and re-draw structure my homework
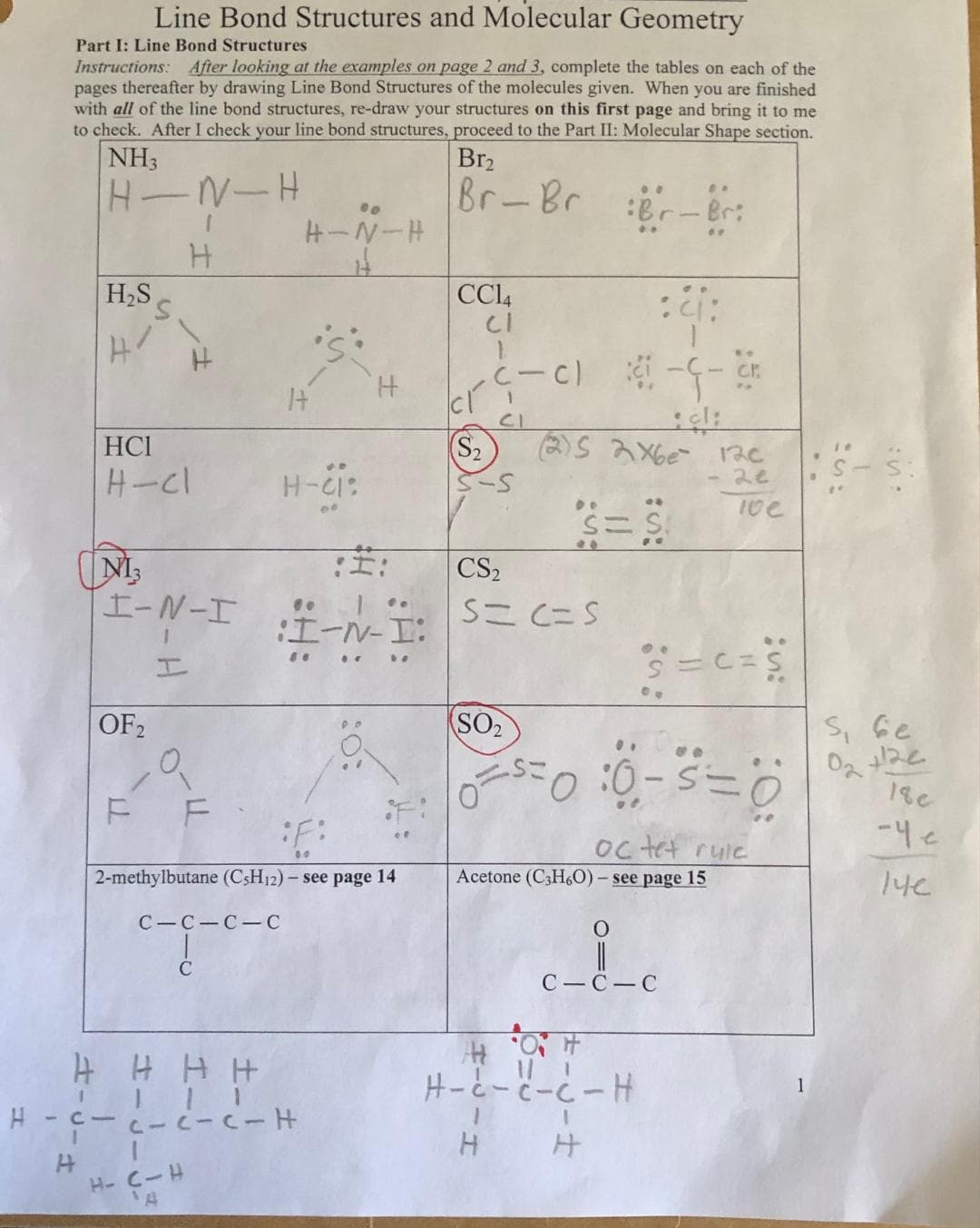
Transcribed Image Text:Part I: Line Bond Structures
Instructions: After looking at the examples on page 2 and 3, complete the tables on each of the
pages thereafter by drawing Line Bond Structures of the molecules given. When you are finished
with all of the line bond structures, re-draw your structures on this first page and bring it to me
to check. After I check your line bond structures, proceed to the Part II: Molecular Shape section.
Br₂
NH3
H-N-H
H
H₂S
1
Line Bond Structures and Molecular Geometry
HCI
H-Cl
NI₂
I-N-I
1
I
OF 2
F F
C-C-C-C
C
00
H-N-H
14
H-C-H
'A
H-CI:
00
:I-N-I:
HHHH
11|1
H-c-c-c-C-H
8.
2-methylbutane (C5H12) - see page 14
H
**
Br-Br Br-Br
CC14
CI
Cí
(S₂
::
C-C) -G-C²
U
CS₂
(2)S 2X6e 12c
- 2€
'S=$
..
S=C=S
..
(SO₂
..
0 = 5 = 0 : 0 - 5 = 0
of
Acetone (C3H6O) - see page 15
O
1
H H
S=C = S
oc tet rule
HO H
H-c-c-c-H
10e
C-C-C
1
**
S, Ge
0₂ +22
18c
-4e
че
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER