PARTIAL PRESSURE T03/S11 Unit conversion The mass percent of a three component gas sample is 14.1% B5H11, 28.6% SO2 and 57.3% COCI2. Calculate the partial pressure (atm) of B5H11 if the total pressure of the sample is 7144 K = C + 273 1 atm = 760 torr Molar Mass (g/mol) B5H11 torr. 65.138 SO2 64.064 COCI2 98.916 the tolerance is +/-2%
PARTIAL PRESSURE T03/S11 Unit conversion The mass percent of a three component gas sample is 14.1% B5H11, 28.6% SO2 and 57.3% COCI2. Calculate the partial pressure (atm) of B5H11 if the total pressure of the sample is 7144 K = C + 273 1 atm = 760 torr Molar Mass (g/mol) B5H11 torr. 65.138 SO2 64.064 COCI2 98.916 the tolerance is +/-2%
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter10: Solids, Liquids, And Phase Transitions
Section: Chapter Questions
Problem 6P
Related questions
Question
please help me. double and triple check your answers, previous tutors got it wrong. this is a practice problem, not for a grade.
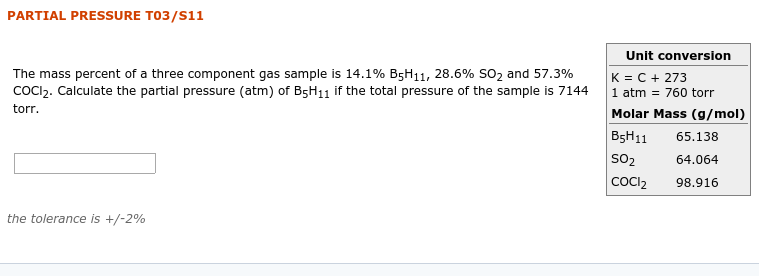
Transcribed Image Text:PARTIAL PRESSURE T03/S11
Unit conversion
The mass percent of a three component gas sample is 14.1% B5H11, 28.6% SO2 and 57.3%
COCI2. Calculate the partial pressure (atm) of B5H11 if the total pressure of the sample is 7144
K = C + 273
1 atm = 760 torr
Molar Mass (g/mol)
B5H11
torr.
65.138
SO2
64.064
COCI2
98.916
the tolerance is +/-2%
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introduction to General, Organic and Biochemistry
Chemistry
ISBN:
9781285869759
Author:
Frederick A. Bettelheim, William H. Brown, Mary K. Campbell, Shawn O. Farrell, Omar Torres
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning