Period 6 metals Period 5 metals (Cs-Hg) (Rb-Cd) 4000 3500 3000 2500 2000 1500 1000 Period 4 metals | (K-Zn) 500 1A 2A 3B 4B 5B 6B 7B 8B 8B 8B 1B 2B Group A Figure 12.21 The melting points of metals from periods 4, 5, and 6. Melting point (kelvin)
Period 6 metals Period 5 metals (Cs-Hg) (Rb-Cd) 4000 3500 3000 2500 2000 1500 1000 Period 4 metals | (K-Zn) 500 1A 2A 3B 4B 5B 6B 7B 8B 8B 8B 1B 2B Group A Figure 12.21 The melting points of metals from periods 4, 5, and 6. Melting point (kelvin)
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter10: Liquids And Solids
Section: Chapter Questions
Problem 60E: Dry ice, CO2(s) , does not melt at atmospheric pressure. It sublimes at a temperature of 78 °C. What...
Related questions
Question
Which element in each period has
the highest melting point? In each
case, is the element you named at
the beginning, middle, or end of its
period?
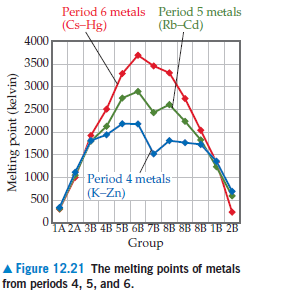
Transcribed Image Text:Period 6 metals Period 5 metals
(Cs-Hg)
(Rb-Cd)
4000
3500
3000
2500
2000
1500
1000
Period 4 metals
| (K-Zn)
500
1A 2A 3B 4B 5B 6B 7B 8B 8B 8B 1B 2B
Group
A Figure 12.21 The melting points of metals
from periods 4, 5, and 6.
Melting point (kelvin)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning
