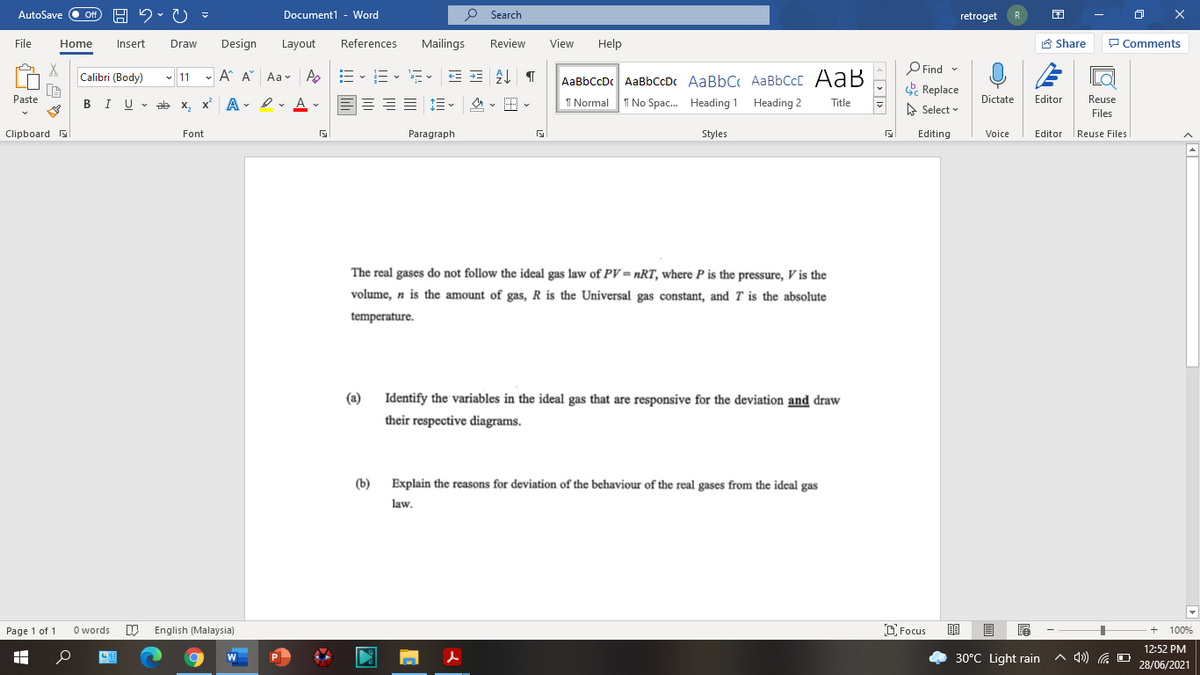
The real gases do not follow the ideal gas law of PV = nRT, where P is the pressure, V is the volume, n is the amount of gas, R is the Universal gas constant, and T is the absolute temperature. (a) Identify the variables in the ideal gas that are responsive for the deviation and draw their respective diagrams.
The real gases do not follow the ideal gas law of PV = nRT, where P is the pressure, V is the volume, n is the amount of gas, R is the Universal gas constant, and T is the absolute temperature. (a) Identify the variables in the ideal gas that are responsive for the deviation and draw their respective diagrams.
Chemistry: The Molecular Science
5th Edition
ISBN:9781285199047
Author:John W. Moore, Conrad L. Stanitski
Publisher:John W. Moore, Conrad L. Stanitski
Chapter10: Fuels, Organic Chemicals, And Polymers
Section: Chapter Questions
Problem 119QRT
Related questions
Question
Transcribed Image Text:AutoSave
Document1 - Word
O Search
ff
retroget
File
Home
Insert
Draw
Design
Layout
References
Mailings
Review
View
Help
A Share
P Comments
P Find -
E Replace
Calibri (Body)
- 11 - A A Aav A
-- v i- v a- v
AaBbCcDc AaBbCcDc AaBbC AaBbCcC AaB
Paste
I U - ab x, x A - I v A v
1 Normal
I No Spac. Heading 1 Heading 2
Dictate
Editor
Reuse
В
Title
A Select
Files
Clipboard
Font
Paragraph
Styles
Editing
Voice
Editor
|Reuse Files
The real gases do not follow the ideal gas law of PV= nRT, where P is the pressure, V is the
volume, n is the amount of gas, R is the Universal gas constant, and T is the absolute
temperature.
(a)
Identify the variables in the ideal gas that are responsive for the deviation and draw
their respective diagrams.
(b)
Explain the reasons for deviation of the behaviour of the real gases from the ideal gas
law.
Page 1 of 1
O words
English (Malaysia)
D Focus
100%
12:52 PM
30°C Light rain
A 4)) G DO
28/06/2021
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781285199023
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning