
Chemistry: Principles and Reactions
8th Edition
ISBN: 9781305079373
Author: William L. Masterton, Cecile N. Hurley
Publisher: Cengage Learning
expand_more
expand_more
format_list_bulleted
Textbook Question
Chapter 12, Problem 74QAP
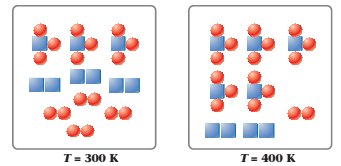
The figures below represent the following reaction at equilibrium at different temperatures.
Expert Solution & Answer
Want to see the full answer?
Check out a sample textbook solution
Chapter 12 Solutions
Chemistry: Principles and Reactions
Ch. 12 - The following data are for the system A(g)2B(g)...Ch. 12 - The following data are for the system A(g)2B(g)...Ch. 12 - Prob. 3QAPCh. 12 - Complete the table below for the reaction...Ch. 12 - Write the equilibrium expressions (K) for the...Ch. 12 - Write the equilibrium expressions (K) for the...Ch. 12 - Write the equilibrium expressions (K) for the...Ch. 12 - Write the equilibrium expressions (K) for the...Ch. 12 - Given the following descriptions of reversible...Ch. 12 - Given the following descriptions of reversible...
Ch. 12 - Write an equation for an equilibrium system that...Ch. 12 - Write a chemical equation for an equilibrium...Ch. 12 - Consider the following reaction at 250C:...Ch. 12 - Consider the following reaction at 1000 C:...Ch. 12 - At 627C, K=0.76 for the reaction...Ch. 12 - At 800C, K=2.2104 for the following reaction...Ch. 12 - Prob. 17QAPCh. 12 - Given the following data at 25C...Ch. 12 - Given the following data at a certain temperature,...Ch. 12 - Consider the following hypothetical reactions and...Ch. 12 - When one mole of carbon disulfide gas reacts with...Ch. 12 - Calculate K for the formation of methyl alcohol at...Ch. 12 - Ammonium carbamate solid (NH4CO2NH2) decomposes at...Ch. 12 - Consider the decomposition at 25C of one mole of...Ch. 12 - Consider the decomposition of ammonium hydrogen...Ch. 12 - A sealed flask has 0.541 atm of SO3 at 1000 K. The...Ch. 12 - A gaseous reaction mixture contains 0.30 atm SO2,...Ch. 12 - For the system PCl5(g)PCl3(g)+Cl2(g)K is 26 at...Ch. 12 - The reversible reaction between hydrogen chloride...Ch. 12 - The reversible reaction between hydrogen chloride...Ch. 12 - A compound, X, decomposes at 131C according to the...Ch. 12 - Consider the following reaction at 75C:...Ch. 12 - Consider the reaction between nitrogen and steam:...Ch. 12 - At 500C, k for the for the formation of ammonia...Ch. 12 - At a certain temperature, K is 4.9 for the...Ch. 12 - At a certain temperature, K=0.29 for the...Ch. 12 - For the reaction N2(g)+2H2O(g)2NO(g)+2H2(g) K is...Ch. 12 - Nitrogen dioxide can decompose to nitrogen oxide...Ch. 12 - Consider the following reaction:...Ch. 12 - Consider the hypothetical reaction at 325C...Ch. 12 - At a certain temperature, the equilibrium constant...Ch. 12 - At 460C, the reaction SO2(g)+NO2(g)NO(g)+SO3(g)...Ch. 12 - Solid ammonium iodide decomposes to ammonia and...Ch. 12 - Consider the following decomposition at 80C....Ch. 12 - Hydrogen cyanide, a highly toxic gas, can...Ch. 12 - At 800 K, hydrogen iodide can decompose into...Ch. 12 - For the following reactions, predict whether the...Ch. 12 - Follow the directions of Question 47 for the...Ch. 12 - Consider the system SO3(g)SO2(g)+12 O2(g)H=98.9kJ...Ch. 12 - Consider the system...Ch. 12 - Predict the direction in which each of the...Ch. 12 - Predict the direction in which each of the...Ch. 12 - At a certain temperature, nitrogen and oxygen...Ch. 12 - Consider the following hypothetical reaction:...Ch. 12 - Iodine chloride decomposes at high temperatures to...Ch. 12 - Sulfur oxychloride, SO2Cl2, decomposes to sulfur...Ch. 12 - For the following reaction C(s)+2H2(g)CH4(g)...Ch. 12 - For the system 2SO3(g)2SO2(g)+O2(g) K=1.32 at 627....Ch. 12 - For a certain reaction, H is +33 kJ. What is the...Ch. 12 - Prob. 60QAPCh. 12 - Hemoglobin (Hb) binds to both oxygen and carbon...Ch. 12 - Mustard gas, used in chemical warfare in World War...Ch. 12 - Prob. 63QAPCh. 12 - For the decomposition of CaCO3 at 900C, K=1.04....Ch. 12 - Isopropyl alcohol is the main ingredient in...Ch. 12 - Consider the equilibrium H2(g)+S(s)H2S(g)When this...Ch. 12 - Prob. 67QAPCh. 12 - The following data apply to the unbalanced...Ch. 12 - Consider the reaction: A(g)+2B(g)+C(s)2D(g)At 25C,...Ch. 12 - For the reaction C(s)+CO2(g)2CO(g) K=168 at 1273...Ch. 12 - Consider the system A(g)+2B(g)+C(g)2D(g)at 25C. At...Ch. 12 - The graph below is similar to that of Figure 12.2....Ch. 12 - Prob. 73QAPCh. 12 - The figures below represent the following reaction...Ch. 12 - Prob. 75QAPCh. 12 - Prob. 76QAPCh. 12 - Consider the following reaction at a certain...Ch. 12 - Prob. 78QAPCh. 12 - Ammonia can decompose into its constituent...Ch. 12 - Hydrogen iodide gas decomposes to hydrogen gas and...Ch. 12 - For the system SO3(g)SO2(g)+12 O2(g)at 1000 K,...Ch. 12 - A student studies the equilibrium I2(g)2I(g)at a...Ch. 12 - At a certain temperature, the reaction...Ch. 12 - Benzaldehyde, a flavoring agent, is obtained by...Ch. 12 - Prob. 85QAPCh. 12 - Prob. 86QAP
Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Similar questions
- The following two diagrams represent the composition of an equilibrium mixture for the reaction A2 + B2 2AB at two different temperatures. Based on the diagrams, is the chemical reaction endothermic or exothermic? Explain your answer using Le Chteliers principle. (A atoms are red and B atoms are green in the diagrams.)arrow_forwardThe equilibrium constant Kc for the synthesis of methanol, CH3OH. CO(g)+2H2(g)CH3OH(g) is 4.3 at 250C and 1.8 at 275C. Is this reaction endothermic or exothermic?arrow_forward12.103 Methanol, CH3OH, can be produced by the reaction of CO with H2, with the liberation of heat. All species in the reaction are gaseous. What effect will each of the following have on the equilibrium concentration of CO? (a) Pressure is increased, (b) volume of the reaction container is decreased, (c) heat is added, (d) the concentration of CO is increased, (e) some methanol is removed from the container, and (f) H2 is added.arrow_forward
- You place 0.600 mol of nitrogen, N2, and 1.800 mol of hydrogen, H2, into a reaction vessel at 450C and 10.0 atm. The reaction is N2(g)+3H2(g)2NH3(g) What is the composition of the equilibrium mixture if you obtain 0.048 mol of ammonia, NH3, from it?arrow_forwardShow that the complete chemical equation, the total ionic equation, and the net ionic equation for the reaction represented by the equation KI(aq)+I2(aq)KI3(aq) give the same expression for the reaction quotient. KI3 is composed of the ions K+ and I3-.arrow_forwardThe following four diagrams represent gaseous equilibrium mixtures for the reaction A2 + B2 2AB at four different temperatures. For which of the diagrams is the numerical value of the equilibrium constant the largest? (A atoms are red and B atoms are green in the various diagrams.)arrow_forward
- Kc = 5.6 1012 at 500 K for the dissociation of iodine molecules to iodine atoms. I2(g) 2 I(g) A mixture has [I2] = 0.020 mol/Land [I] = 2.0 108 mol/L. Is the reaction at equilibrium (at 500 K)? If not, which way must the reaction proceed to reach equilibrium?arrow_forwardAt a certain temperature, K=0.29 for the decomposition of two moles of iodine trichloride, ICl3(s), to chlorine and iodine gases. The partial pressure of chlorine gas at equilibrium is three times that of iodine gas. What are the partial pressures of iodine and chlorine at equilibrium?arrow_forwardThe decomposition of PCl5(g) to form PCl3(g) and Cl2(g) has Kc = 33.3 at a high temperature. If the initial concentration of PCl5 is 0.1000 M, what are the equilibrium concentrations of the reactants and products?arrow_forward
- For the reaction N2(g)+3H2(g)2NH3(g) show that Kc = Kp(RT)2 Do not use the formula Kp = Kc(RT)5n given in the text. Start from the fact that Pi = [i]RT, where Pi is the partial pressure of substance i and [i] is its molar concentration. Substitute into Kc.arrow_forwardSuppose a reaction has the equilibrium constant K = 1.3 108. What does the magnitude of this constant tell you about the relative concentrations of products and reactants that will be present once equilibrium is reached? Is this reaction likely to be a good source of the products?arrow_forwardA solution is prepared by dissolving 0.050 mol of diiodocyclohexane, C5H10I2, in the solvent CCl4.The total solution volume is 1.00 L When the reaction C6H10I2 C6H10 + I2 has come to equilibrium at 35 C, the concentration of I2 is 0.035 mol/L. (a) What are the concentrations of C6H10I2 and C6H10 at equilibrium? (b) Calculate Kc, the equilibrium constant.arrow_forward
arrow_back_ios
SEE MORE QUESTIONS
arrow_forward_ios
Recommended textbooks for you
 General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
General, Organic, and Biological ChemistryChemistryISBN:9781285853918Author:H. Stephen StokerPublisher:Cengage Learning
 ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning
ChemistryChemistryISBN:9781305957404Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCostePublisher:Cengage Learning Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning
Chemistry: An Atoms First ApproachChemistryISBN:9781305079243Author:Steven S. Zumdahl, Susan A. ZumdahlPublisher:Cengage Learning Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning
Chemistry for Engineering StudentsChemistryISBN:9781337398909Author:Lawrence S. Brown, Tom HolmePublisher:Cengage Learning General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning
General Chemistry - Standalone book (MindTap Cour...ChemistryISBN:9781305580343Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; DarrellPublisher:Cengage Learning

General, Organic, and Biological Chemistry
Chemistry
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:Cengage Learning


Chemistry
Chemistry
ISBN:9781305957404
Author:Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:9781305079243
Author:Steven S. Zumdahl, Susan A. Zumdahl
Publisher:Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:9781337398909
Author:Lawrence S. Brown, Tom Holme
Publisher:Cengage Learning

General Chemistry - Standalone book (MindTap Cour...
Chemistry
ISBN:9781305580343
Author:Steven D. Gammon, Ebbing, Darrell Ebbing, Steven D., Darrell; Gammon, Darrell Ebbing; Steven D. Gammon, Darrell D.; Gammon, Ebbing; Steven D. Gammon; Darrell
Publisher:Cengage Learning
Chemical Equilibria and Reaction Quotients; Author: Professor Dave Explains;https://www.youtube.com/watch?v=1GiZzCzmO5Q;License: Standard YouTube License, CC-BY