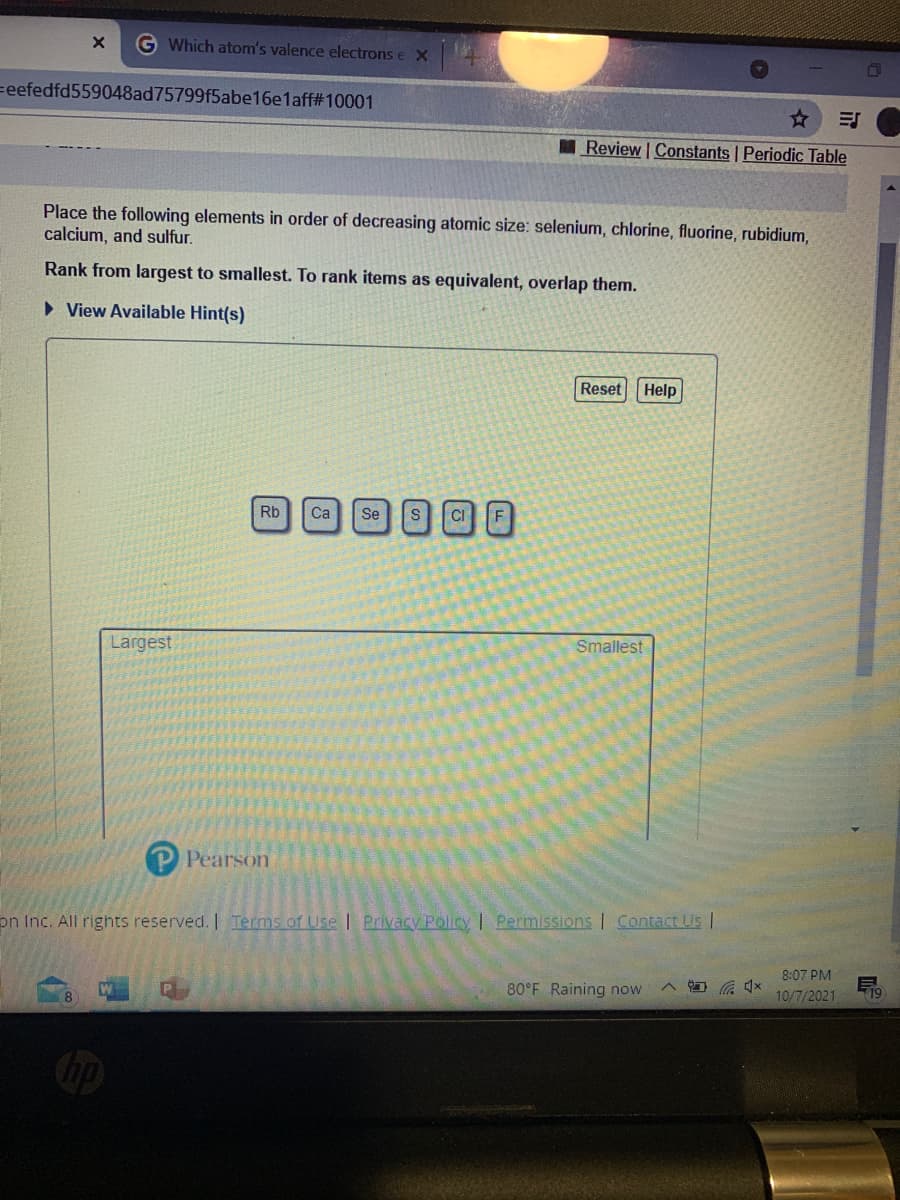
Place the following elements in order of decreasing atomic size: selenium, chlorine, fluorine, rubidium, calcium, and sulfur. Rank from largest to smallest. To rank items as equivalent, overlap them. • View Available Hint(s) Reset Help S CF Rb Ca Se
Place the following elements in order of decreasing atomic size: selenium, chlorine, fluorine, rubidium, calcium, and sulfur. Rank from largest to smallest. To rank items as equivalent, overlap them. • View Available Hint(s) Reset Help S CF Rb Ca Se
Introductory Chemistry: An Active Learning Approach
6th Edition
ISBN:9781305079250
Author:Mark S. Cracolice, Ed Peters
Publisher:Mark S. Cracolice, Ed Peters
Chapter11: Atomic Theory :the Quantum Model Of The Atom
Section: Chapter Questions
Problem 11.5TC
Related questions
Question
Transcribed Image Text:G Which atom's valence electrons e X
=eefedfd559048ad75799f5abe16e1aff#10001
Review | Constants Periodic Table
Place the following elements in order of decreasing atomic size: selenium, chlorine, fluorine, rubidium,
calcium, and sulfur.
Rank from largest to smallest. To rank items as equivalent, overlap them.
• View Available Hint(s)
Reset Help
Rb
Ca
Se
CI
Largest
Smallest
Pearson
on Inc. All rights reserved. | Terms of Use | Privacy Policy | Permissions | Contact Us |
8:07 PM
8.
80°F Raining now
10/7/2021
19
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps

Recommended textbooks for you

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Introductory Chemistry: An Active Learning Approa…
Chemistry
ISBN:
9781305079250
Author:
Mark S. Cracolice, Ed Peters
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning