Chemistry & Chemical Reactivity
9th Edition
ISBN:9781133949640
Author:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Chapter14: Chemical Kinetics: The Rates Of Chemical Reactions
Section: Chapter Questions
Problem 5PS: Experimental data are listed here for the reaction A 2 B. (a) Prepare a graph from these data;...
Related questions
Question
Please help need help with 1 to 4
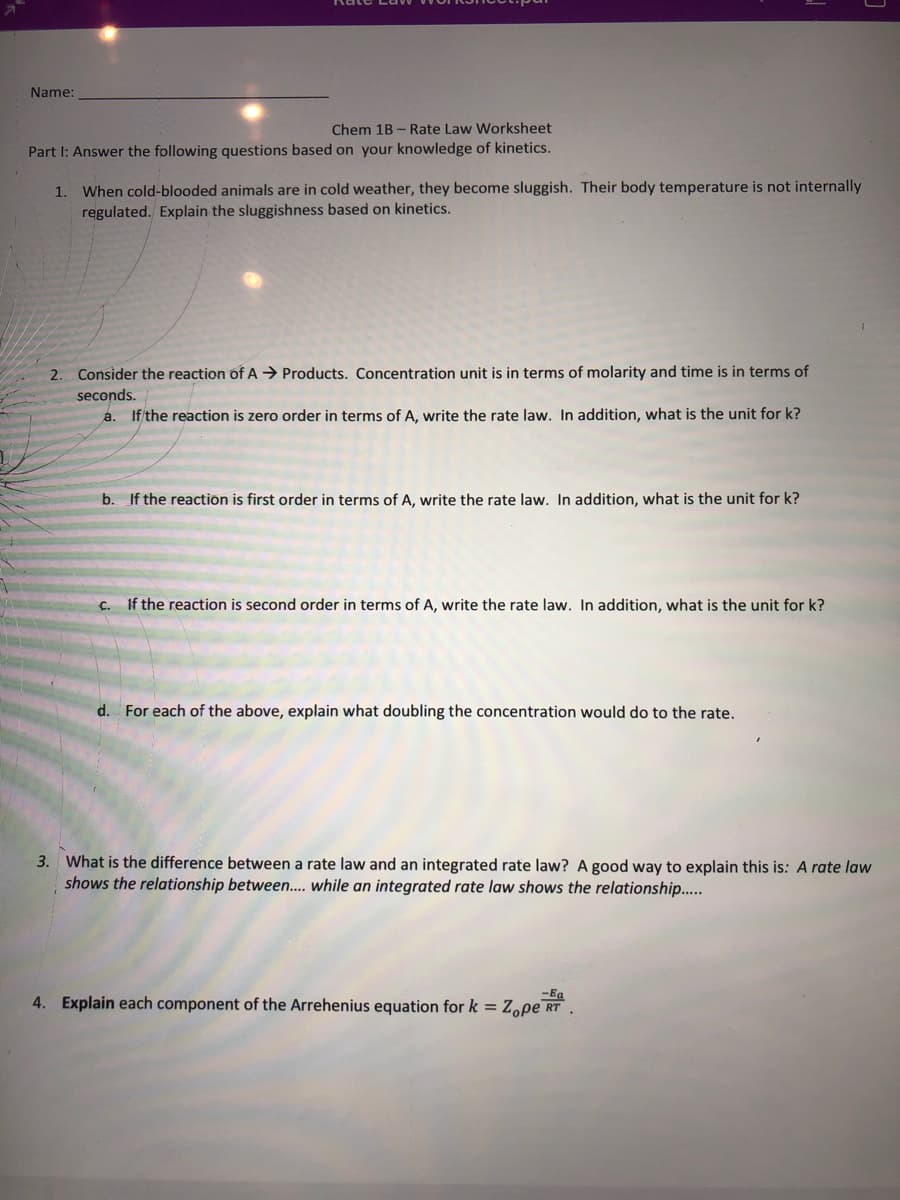
Transcribed Image Text:Name:
Chem 1B - Rate Law Worksheet
Part I: Answer the following questions based on your knowledge of kinetics.
When cold-blooded animals are in cold weather, they become sluggish. Their body temperature is not internally
regulated. Explain the sluggishness based on kinetics.
1.
2. Consider the reaction of A → Products. Concentration unit is in terms of molarity and time is in terms of
seconds.
a. If the reaction is zero order in terms of A, write the rate law. In addition, what is the unit for k?
b. If the reaction is first order in terms of A, write the rate law. In addition, what is the unit for k?
C.
if the reaction is second order in terms of A, write the rate law. In addition, what is the unit for k?
d.
For each of the above, explain what doubling the concentration would do to the rate.
3. What is the difference between a rate law and an integrated rate law? A good way to explain this is: A rate law
shows the relationship between.. while an integrated rate law shows the relationship..
-Ba
4. Explain each component of the Arrehenius equation for k = Zope rt .
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781133949640
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning


Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry for Engineering Students
Chemistry
ISBN:
9781337398909
Author:
Lawrence S. Brown, Tom Holme
Publisher:
Cengage Learning

Chemistry & Chemical Reactivity
Chemistry
ISBN:
9781337399074
Author:
John C. Kotz, Paul M. Treichel, John Townsend, David Treichel
Publisher:
Cengage Learning