Problem 19.31 Consider the following two-step process. Heat is allowed to flow out of an ideal gas at constant volume so that its AEint = pressure drops from 2.2 atm to 1.4 atm. Then the gas expands at constant pressure, from a volume of 5.9 L to 9.3 L, where the temperature reaches its original value. (See figure(Figure 1)). Submit Request Answer Part C Calculate the total heat flow into or out of the gas. Figure < 1 of 1 Express your answer using two significant figures. П 2.2 atm |Q = J 1.4 atm Submit Request Answer Part D Problem 19.31 Consider the following two-step process. Heat is allowed to flow out of an ideal gas at constant volume so that its pressure drops from 2.2 atm to 1.4 atm. Then the gas expands at constant pressure, from a volume of 5.9 L to 9.3 L, where the temperature reaches its original value. (See figure(Figure 1)). Part A Calculate the total work done by the gas in the process. Express your answer using two significant figures. Hν ΑΣφ Figure <) 1 of 1 Submit Request Answer Part B 2.2 atm Calculate the change in internal energy of the gas in the process. 1.4 atm ηVα ΑΣφ AEint = 5.9 L 9.3 L Submit Request Answer
Problem 19.31 Consider the following two-step process. Heat is allowed to flow out of an ideal gas at constant volume so that its AEint = pressure drops from 2.2 atm to 1.4 atm. Then the gas expands at constant pressure, from a volume of 5.9 L to 9.3 L, where the temperature reaches its original value. (See figure(Figure 1)). Submit Request Answer Part C Calculate the total heat flow into or out of the gas. Figure < 1 of 1 Express your answer using two significant figures. П 2.2 atm |Q = J 1.4 atm Submit Request Answer Part D Problem 19.31 Consider the following two-step process. Heat is allowed to flow out of an ideal gas at constant volume so that its pressure drops from 2.2 atm to 1.4 atm. Then the gas expands at constant pressure, from a volume of 5.9 L to 9.3 L, where the temperature reaches its original value. (See figure(Figure 1)). Part A Calculate the total work done by the gas in the process. Express your answer using two significant figures. Hν ΑΣφ Figure <) 1 of 1 Submit Request Answer Part B 2.2 atm Calculate the change in internal energy of the gas in the process. 1.4 atm ηVα ΑΣφ AEint = 5.9 L 9.3 L Submit Request Answer
Chapter3: The First Law Of Thermodynamics
Section: Chapter Questions
Problem 17CQ: There is no change in the internal of an ideal gas undergoing an isothermal process since the...
Related questions
Question
Please help me there are multiple parts, double check your answer as previous tutors got it wrong.
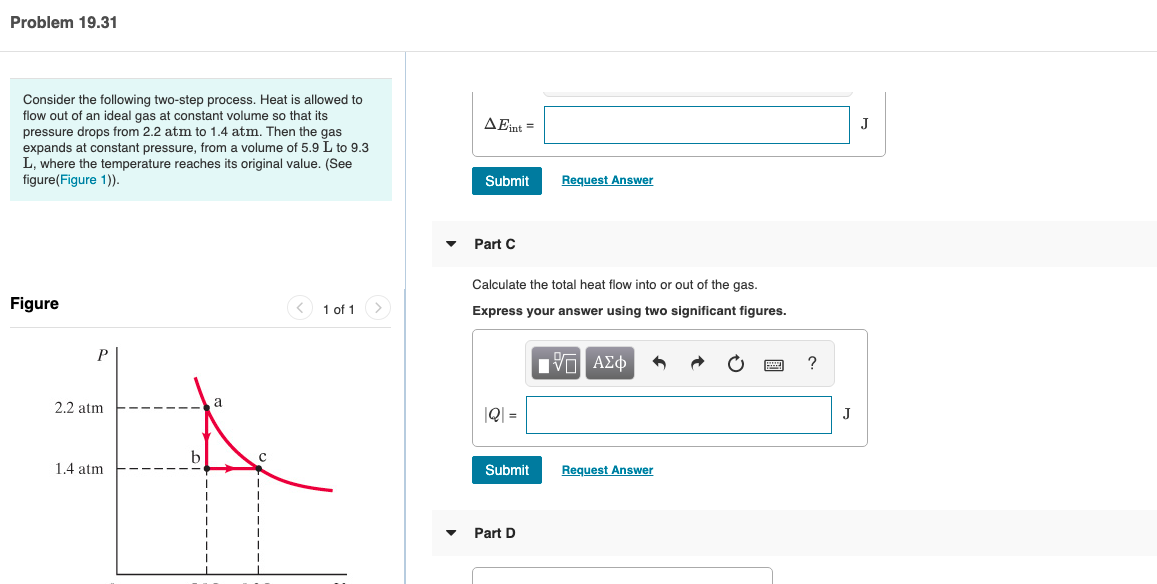
Transcribed Image Text:Problem 19.31
Consider the following two-step process. Heat is allowed to
flow out of an ideal gas at constant volume so that its
AEint =
pressure drops from 2.2 atm to 1.4 atm. Then the gas
expands at constant pressure, from a volume of 5.9 L to 9.3
L, where the temperature reaches its original value. (See
figure(Figure 1)).
Submit
Request Answer
Part C
Calculate the total heat flow into or out of the gas.
Figure
< 1 of 1
Express your answer using two significant figures.
П
2.2 atm
|Q =
J
1.4 atm
Submit
Request Answer
Part D
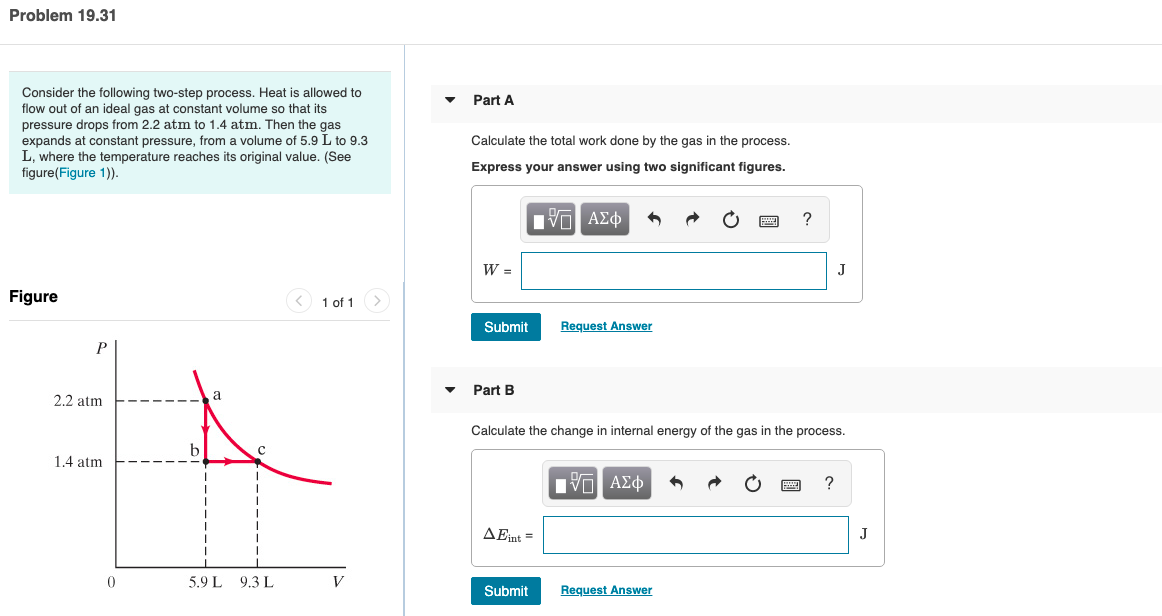
Transcribed Image Text:Problem 19.31
Consider the following two-step process. Heat is allowed to
flow out of an ideal gas at constant volume so that its
pressure drops from 2.2 atm to 1.4 atm. Then the gas
expands at constant pressure, from a volume of 5.9 L to 9.3
L, where the temperature reaches its original value. (See
figure(Figure 1)).
Part A
Calculate the total work done by the gas in the process.
Express your answer using two significant figures.
Hν ΑΣφ
Figure
<) 1 of 1
Submit
Request Answer
Part B
2.2 atm
Calculate the change in internal energy of the gas in the process.
1.4 atm
ηVα ΑΣφ
AEint =
5.9 L 9.3 L
Submit
Request Answer
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 4 steps with 4 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, physics and related others by exploring similar questions and additional content below.Recommended textbooks for you


College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College


College Physics
Physics
ISBN:
9781938168000
Author:
Paul Peter Urone, Roger Hinrichs
Publisher:
OpenStax College