PROBLEM SOLVING: REPORT QUANTITIES IN THEIR PROPER SIGNIFICANT FIGURES. NO NEED TO TYPE UNITS IN YOUR ANSWER. DO NOT ROUND OFF IN BETWEEN CALCULATIONS. The %purity of a powdered crude sample of Na2CO3 containing only inert impurities is to be determined by reacting 225.0 mg of the crude sample to 10.0 mL of 3.00 M HCI solution, and bubbling the resulting CO2(g) product in water that is at exactly 29 °C. After the reaction has completed, the level of the liquid inside the eudiometer rests 4.30 cm above the water level in the beaker. The graduation on the eudiometer indicates that the trapped gas is 44.37 mL. The experiment was done under a barometric pressure of 755.2 tor. Collection of CO2 *that has displaced Eudiometer filled with the water water Burette Clamp Temperature Vapor Pressure of Water, PH‚0 ("C) 16 17 Stopper or Cork (Torr) 13.5 14.5 15.5 16.5 17.5 Bubbles of Liberated CO2" 18 19 20 Flask Tubing Ring Stand 21 22 23 18.7 19.3 21.1 22.4 24 23.8 25.2 26.7 28.3 30.0 Sample + HCI 25 26 27 28 29 500ml Beaker filled with water
PROBLEM SOLVING: REPORT QUANTITIES IN THEIR PROPER SIGNIFICANT FIGURES. NO NEED TO TYPE UNITS IN YOUR ANSWER. DO NOT ROUND OFF IN BETWEEN CALCULATIONS. The %purity of a powdered crude sample of Na2CO3 containing only inert impurities is to be determined by reacting 225.0 mg of the crude sample to 10.0 mL of 3.00 M HCI solution, and bubbling the resulting CO2(g) product in water that is at exactly 29 °C. After the reaction has completed, the level of the liquid inside the eudiometer rests 4.30 cm above the water level in the beaker. The graduation on the eudiometer indicates that the trapped gas is 44.37 mL. The experiment was done under a barometric pressure of 755.2 tor. Collection of CO2 *that has displaced Eudiometer filled with the water water Burette Clamp Temperature Vapor Pressure of Water, PH‚0 ("C) 16 17 Stopper or Cork (Torr) 13.5 14.5 15.5 16.5 17.5 Bubbles of Liberated CO2" 18 19 20 Flask Tubing Ring Stand 21 22 23 18.7 19.3 21.1 22.4 24 23.8 25.2 26.7 28.3 30.0 Sample + HCI 25 26 27 28 29 500ml Beaker filled with water
Chapter2: Crystallization
Section: Chapter Questions
Problem 3Q
Related questions
Question
- Write the balanced chemical equation of the reaction in your solutions sheet. What is the sum of all the coefficients of the balanced chemical equation?
- Determine the pressure of the trapped gas inside the eudiometer in mmHg.
- Determine the partial pressure of the collected CO2 in mm Hg.
- How many millimoles of CO2 was collected?
- What is the %purity of the sample to the nearest whole number (disregard the % symbol)?
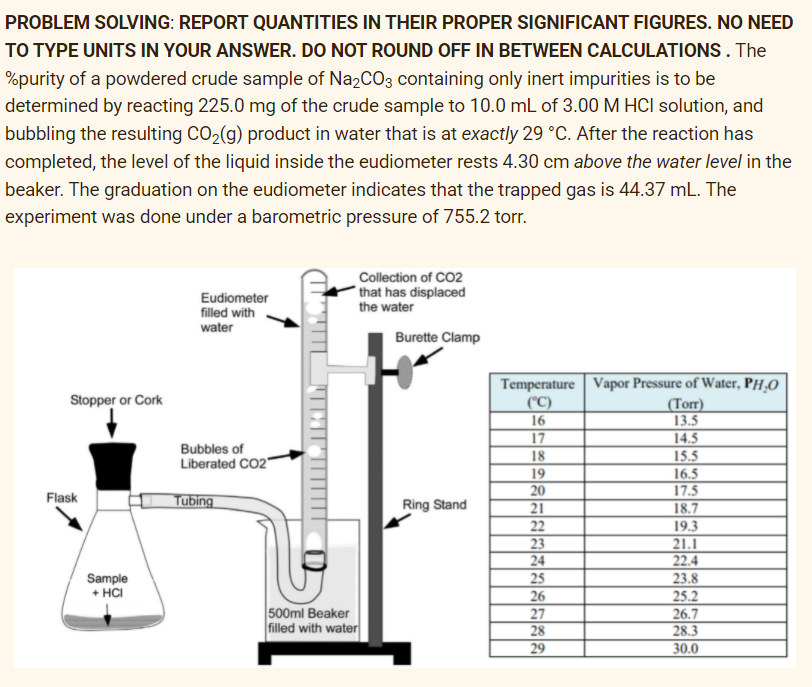
Transcribed Image Text:PROBLEM SOLVING: REPORT QUANTITIES IN THEIR PROPER SIGNIFICANT FIGURES. NO NEED
TO TYPE UNITS IN YOUR ANSWER. DO NOT ROUND OFF IN BETWEEN CALCULATIONS. The
%purity of a powdered crude sample of Na,CO3 containing only inert impurities is to be
determined by reacting 225.0 mg of the crude sample to 10.0 mL of 3.00 M HCI solution, and
bubbling the resulting CO2(g) product in water that is at exactly 29 °C. After the reaction has
completed, the level of the liquid inside the eudiometer rests 4.30 cm above the water level in the
beaker. The graduation on the eudiometer indicates that the trapped gas is 44.37 mL. The
experiment was done under a barometric pressure of 755.2 torr.
Collection of CO2
that has displaced
Eudiometer
filled with
water
the water
Burette Clamp
Temperature Vapor Pressure of Water, PH,O
|("C)
16
Stopper or Cork
(Torr)
13.5
14.5
17
Bubbles of
Liberated CO2"
18
15.5
19
20
21
22
16.5
17.5
18.7
Flask
Tubing
Ring Stand
23
24
25
26
27
28
19.3
21.1
22.4
23.8
25.2
26.7
28.3
Sample
+ HCI
500ml Beaker
filled with water
29
30.0
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT

EBK A SMALL SCALE APPROACH TO ORGANIC L
Chemistry
ISBN:
9781305446021
Author:
Lampman
Publisher:
CENGAGE LEARNING - CONSIGNMENT