Pure Homogeneous Heterogeneous Substance Substance Mixture Mixture Concrete Seawater Magnesium Gasoline Air Nitrogen Iodine crystals Blue-cheese salad dressing
Pure Homogeneous Heterogeneous Substance Substance Mixture Mixture Concrete Seawater Magnesium Gasoline Air Nitrogen Iodine crystals Blue-cheese salad dressing
General, Organic, and Biological Chemistry
7th Edition
ISBN:9781285853918
Author:H. Stephen Stoker
Publisher:H. Stephen Stoker
Chapter7: Gases, Liquids, And Solids
Section: Chapter Questions
Problem 7.8EP
Related questions
Question
100%
Please Help
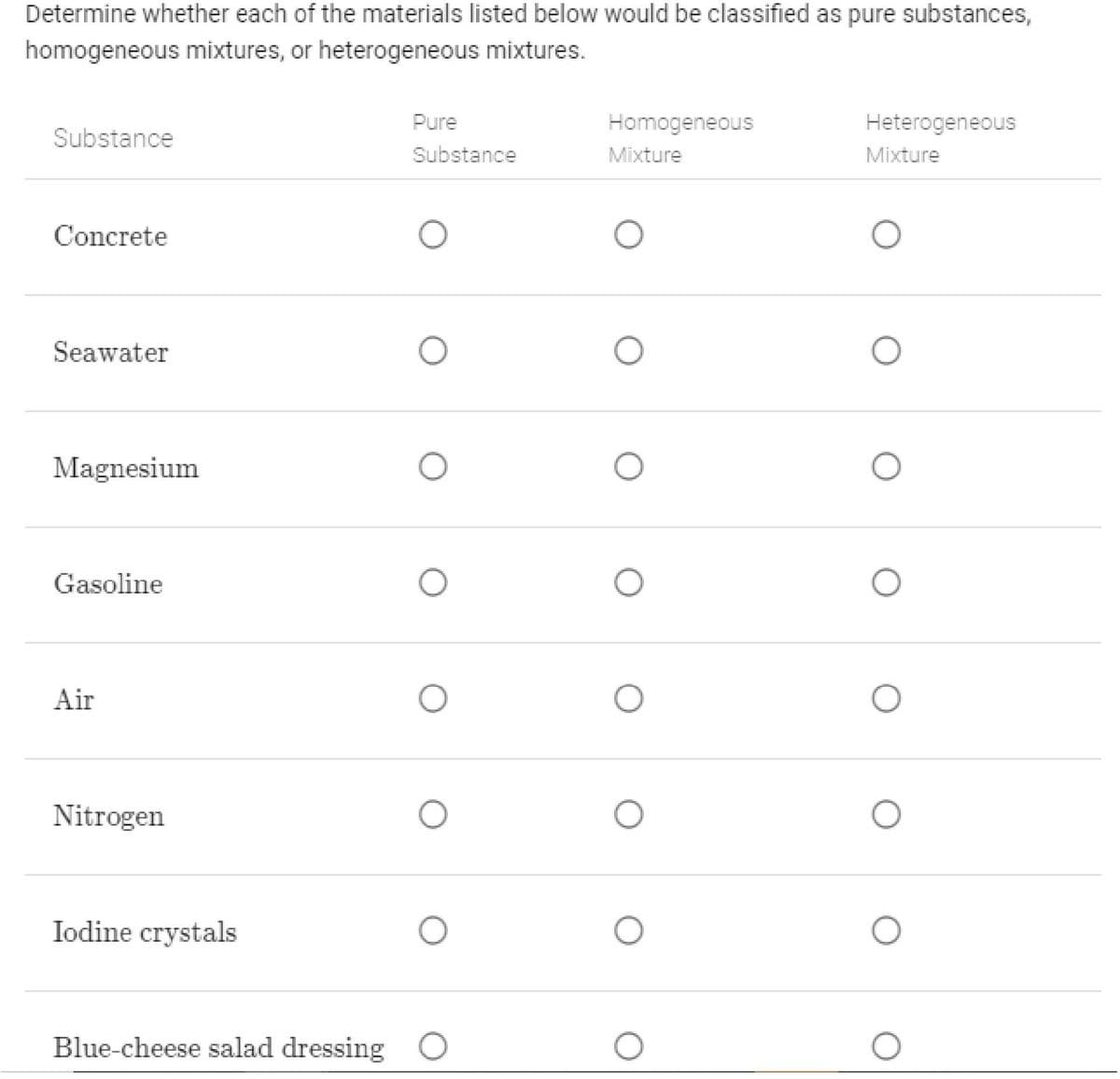
Transcribed Image Text:Determine whether each of the materials listed below would be classified as pure substances,
homogeneous mixtures, or heterogeneous mixtures.
Pure
Homogeneous
Heterogeneous
Substance
Substance
Mixture
Mixture
Concrete
Seawater
Magnesium
Gasoline
Air
Nitrogen
Iodine crystals
Blue-cheese salad dressing O
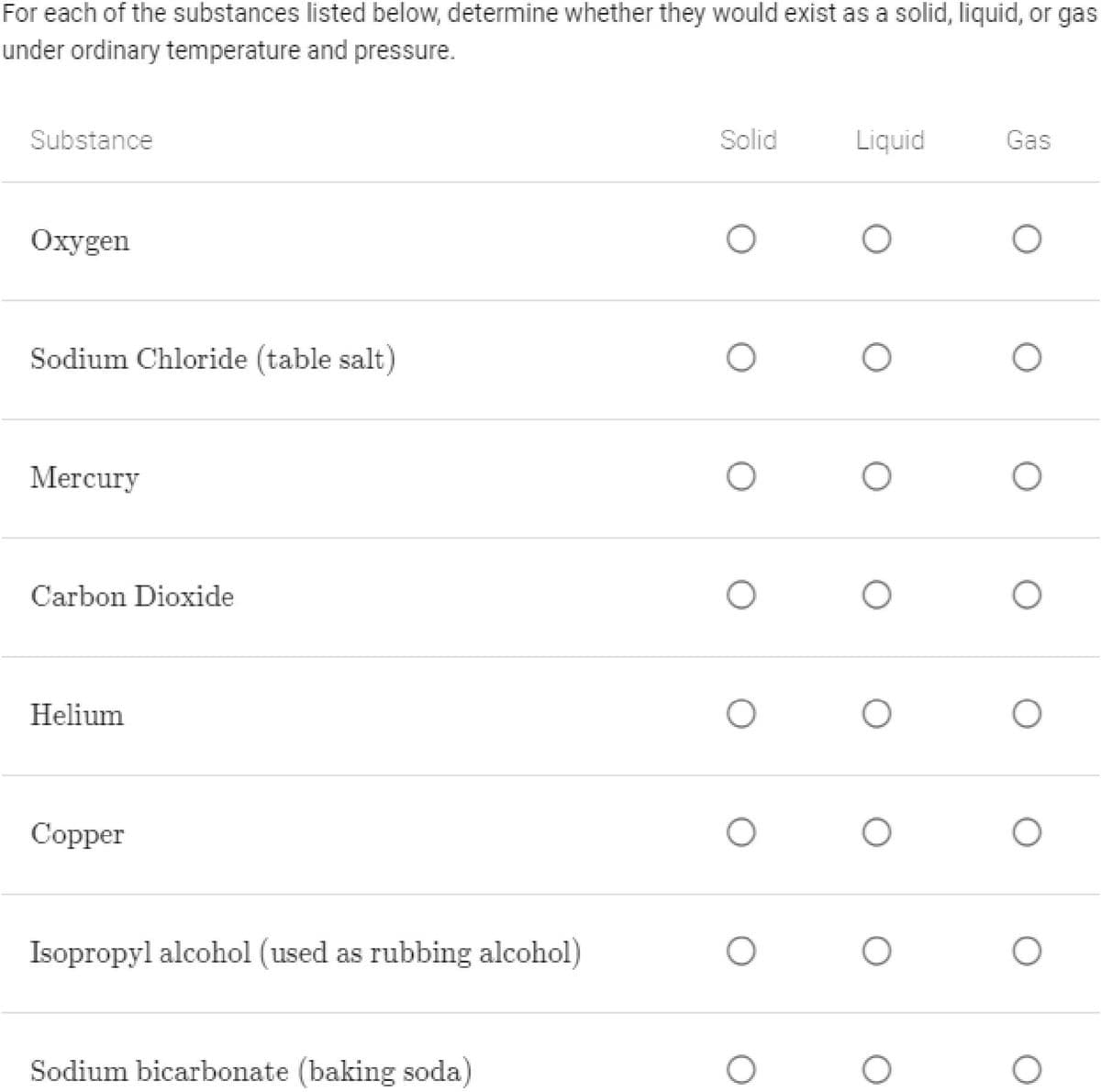
Transcribed Image Text:For each of the substances listed below, determine whether they would exist as a solid, liquid, or gas
under ordinary temperature and pressure.
Substance
Solid
Liquid
Gas
Oxygen
Sodium Chloride (table salt)
Mercury
Carbon Dioxide
Helium
Copper
Isopropyl alcohol (used as rubbing alcohol)
Sodium bicarbonate (baking soda)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 3 steps

Recommended textbooks for you

General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning


General, Organic, and Biological Chemistry
Chemistry
ISBN:
9781285853918
Author:
H. Stephen Stoker
Publisher:
Cengage Learning

World of Chemistry, 3rd edition
Chemistry
ISBN:
9781133109655
Author:
Steven S. Zumdahl, Susan L. Zumdahl, Donald J. DeCoste
Publisher:
Brooks / Cole / Cengage Learning


Chemistry for Today: General, Organic, and Bioche…
Chemistry
ISBN:
9781305960060
Author:
Spencer L. Seager, Michael R. Slabaugh, Maren S. Hansen
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning