Q1: |The results of a photoelectric experiment comparing two different metals (A ar B) are shown below. A B Frequency of light In the experiment, the KE of the photoelectrons is plotted vs. the frequency of the light used for each of the metals. Which metal has the greater p? Group of answer choices: B/cannot be determined/A Photoelectron KE
Q1: |The results of a photoelectric experiment comparing two different metals (A ar B) are shown below. A B Frequency of light In the experiment, the KE of the photoelectrons is plotted vs. the frequency of the light used for each of the metals. Which metal has the greater p? Group of answer choices: B/cannot be determined/A Photoelectron KE
Chemistry by OpenStax (2015-05-04)
1st Edition
ISBN:9781938168390
Author:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Chapter6: Electronic Structure And Periodic Properties Of Elements
Section: Chapter Questions
Problem 15E: What is the threshold frequency for sodium metal if a photon with frequency 6.661014s1 ejects an...
Related questions
Question
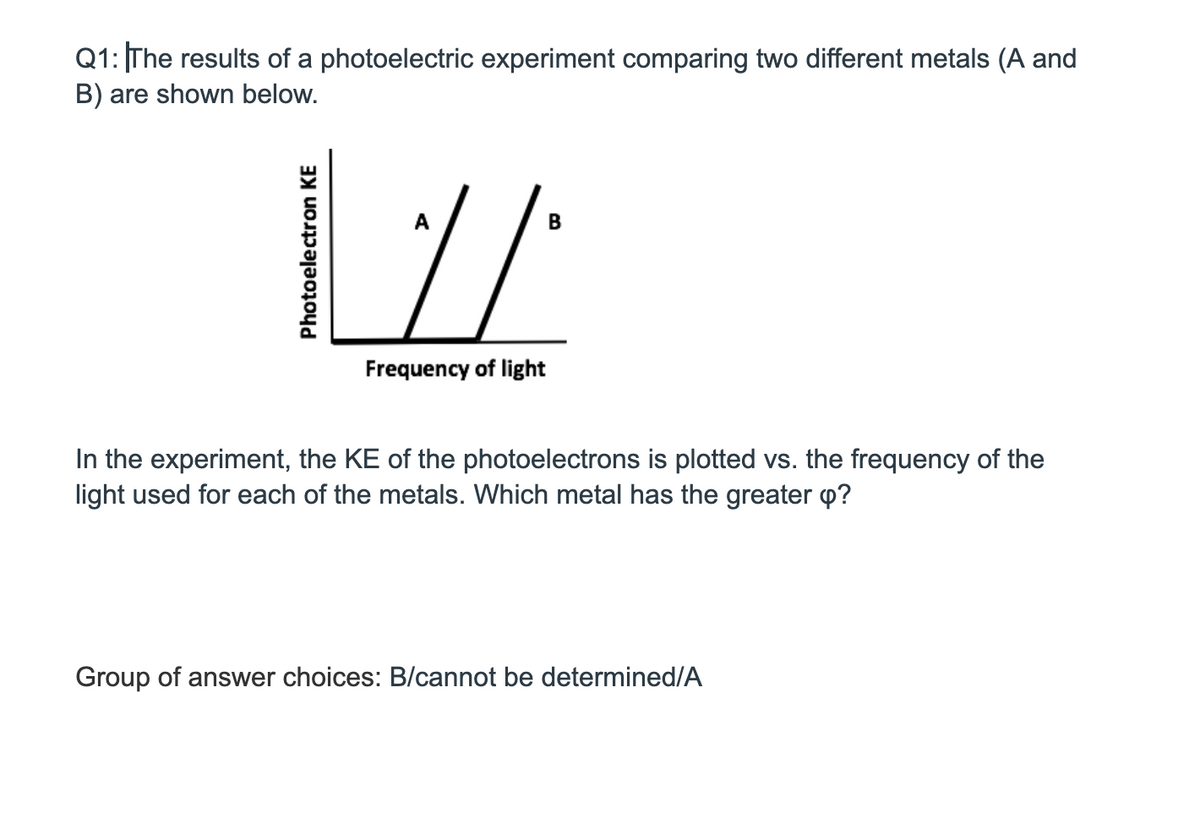
Transcribed Image Text:Q1: The results of a photoelectric experiment comparing two different metals (A and
B) are shown below.
A
B
Frequency of light
In the experiment, the KE of the photoelectrons is plotted vs. the frequency of the
light used for each of the metals. Which metal has the greater p?
Group of answer choices: B/cannot be determined/A
Photoelectron KE
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: The Molecular Science
Chemistry
ISBN:
9781285199047
Author:
John W. Moore, Conrad L. Stanitski
Publisher:
Cengage Learning

Introductory Chemistry: A Foundation
Chemistry
ISBN:
9781337399425
Author:
Steven S. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning