Q13. A hydrocarbon with n x-electrons is proposed as a model compound for an application of the particle-in-a-square-box model. If the lowest possible total energy of the system is 24e, what is n? F) 6 B) 2 C) 3 D) 4 E) 5 A) 1 neighb
Q13. A hydrocarbon with n x-electrons is proposed as a model compound for an application of the particle-in-a-square-box model. If the lowest possible total energy of the system is 24e, what is n? F) 6 B) 2 C) 3 D) 4 E) 5 A) 1 neighb
Physical Chemistry
2nd Edition
ISBN:9781133958437
Author:Ball, David W. (david Warren), BAER, Tomas
Publisher:Ball, David W. (david Warren), BAER, Tomas
Chapter14: Rotational And Vibrational Spectroscopy
Section: Chapter Questions
Problem 14.45E: Determine the number of total degrees of freedom and the number of vibrational degrees of freedom...
Related questions
Question
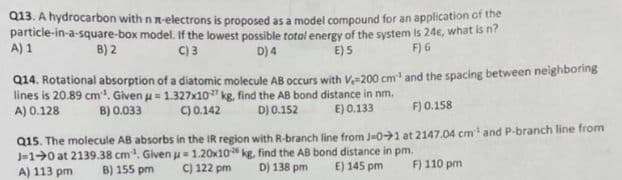
Transcribed Image Text:Q13. A hydrocarbon with n n-electrons is proposed as a model compound for an application of the
particle-in-a-square-box model. If the lowest possible total energy of the system is 24€, what is n?
D) 4
B) 2
C) 3
E) 5
F) 6
A) 1
Q14. Rotational absorption of a diatomic molecule AB occurs with V-200 cm³ and the spacing between neighboring
lines is 20.89 cm³. Given u = 1.327x10 kg, find the AB bond distance in nm.
A) 0.128
D) 0.152
B) 0.033
C) 0.142
E) 0.133
F) 0.158
Q15. The molecule AB absorbs in the IR region with R-branch line from J-01 at 2147.04 cm¹ and P-branch line from
J-10 at 2139.38 cm³. Given u 1.20x10
kg, find the AB bond distance in pm.
D) 138 pm E) 145 pm
A) 113 pm B) 155 pm
C) 122 pm
F) 110 pm
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning

Physical Chemistry
Chemistry
ISBN:
9781133958437
Author:
Ball, David W. (david Warren), BAER, Tomas
Publisher:
Wadsworth Cengage Learning,

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning

Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning
