Q8.4 Structure CH3 CH3 ВЕ .C -E D I II What substituent is positioned at B on Structure II? O -H O -CH3 Q8.5 Structure CH3 CH3 В ВЕ .C A -E H3C A I II What substituent is positioned at C on Structure II? O H O -CH3 Q8.6 Structure CH3 CH3 B E A H3C A F, F I II What substituent is positioned at E on Structure II? O -H -CH3
Q8.4 Structure CH3 CH3 ВЕ .C -E D I II What substituent is positioned at B on Structure II? O -H O -CH3 Q8.5 Structure CH3 CH3 В ВЕ .C A -E H3C A I II What substituent is positioned at C on Structure II? O H O -CH3 Q8.6 Structure CH3 CH3 B E A H3C A F, F I II What substituent is positioned at E on Structure II? O -H -CH3
Chapter3: Organic Compounds: Alkanes And Their Stereochemistry
Section3.SE: Something Extra
Problem 54AP: In the next chapter we'll look at cycloalkanes—saturated cyclic hydrocarbons—and we’ll see...
Related questions
Question
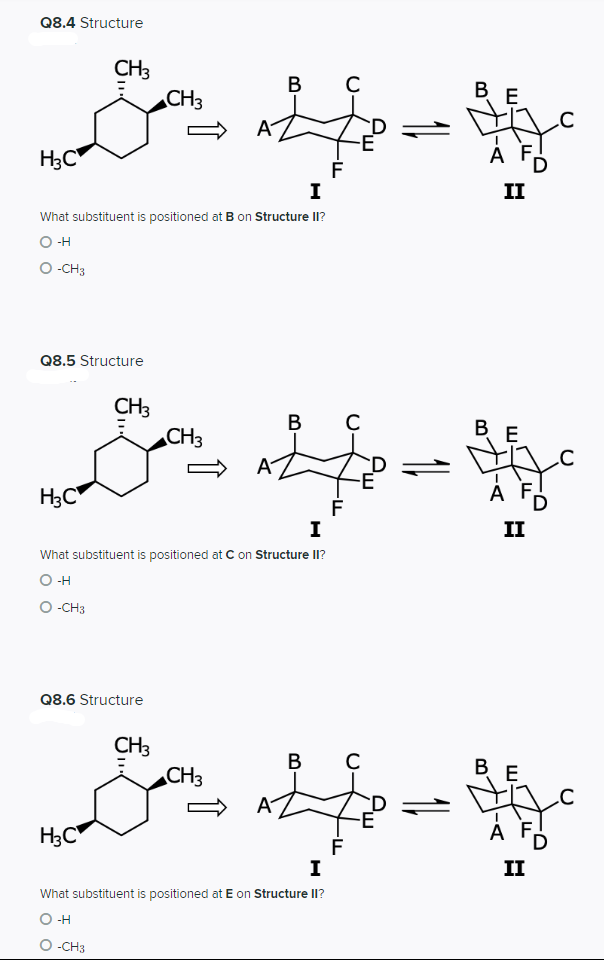
Transcribed Image Text:Q8.4 Structure
CH3
CH3
В
B E
A
H3C
A
I
II
What substituent is positioned at B on Structure II?
O -H
O -CH3
Q8.5 Structure
CH3
CH3
В
A
H3C
ÀF,
I
II
What substituent is positioned at C on Structure II?
O H
O -CH3
Q8.6 Structure
CH3
CH3
В
C
В Е
A
H3C"
A F
F
I
II
What substituent is positioned at E on Structure II?
O -H
-CH3
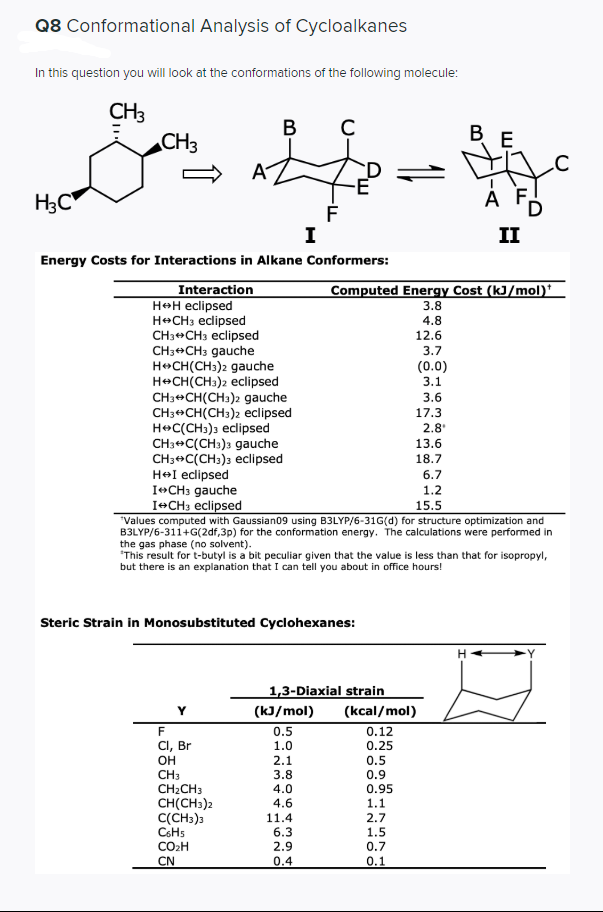
Transcribed Image Text:Q8 Conformational Analysis of Cycloalkanes
In this question you will look at the conformations of the following molecule:
CH3
C
В F
CH3
D
-E
A
H3C
F
I
II
Energy Costs for Interactions in Alkane Conformers:
Computed Energy Cost (kJ/mol)*
3.8
Interaction
H+H eclipsed
H+CH3 eclipsed
CH3+CH3 eclipsed
CH3+CH3 gauche
H»CH(CH3)2 gauche
H CH(CH:)2 eclipsed
CH3+CH(CH3)2 gauche
CH3+CH(CH3)2 eclipsed
HeC(CH:)3 eclipsed
CH3+C(CH3)3 gauche
CH3+C(CH:)3 eclipsed
H+I eclipsed
I+CH3 gauche
I+CH3 eclipsed
"Values computed with Gaussian09 using B3LYP/6-31G(d) for structure optimization and
B3LYP/6-311+G(2df,3p) for the conformation energy. The calculations were performed in
the gas phase (no solvent).
*This result for t-butyl is a bit peculiar given that the value is less than that for isopropyl,
but there is an explanation that I can tell you about in office hours!
4.8
12.6
3.7
(0.0)
3.1
3.6
17.3
2.8
13.6
18.7
6.7
1.2
15.5
Steric Strain in Monosubstituted Cyclohexanes:
1,3-Diaxial strain
Y
(kJ/mol)
(kcal/mol)
F
0.5
0.12
CI, Br
он
CH3
CH2CH3
CH(CH3)2
C(CH3)3
CSHS
CO2H
CN
1.0
0.25
2.1
0.5
0.9
0.95
1.1
2.7
1.5
0.7
0.1
3.8
4.0
4.6
11.4
6.3
2.9
0.4
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
Step by step
Solved in 2 steps with 2 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning


Organic Chemistry
Chemistry
ISBN:
9781305580350
Author:
William H. Brown, Brent L. Iverson, Eric Anslyn, Christopher S. Foote
Publisher:
Cengage Learning

Organic Chemistry: A Guided Inquiry
Chemistry
ISBN:
9780618974122
Author:
Andrei Straumanis
Publisher:
Cengage Learning