Q9 9a: Rank the water solubility of the following compounds: al H3C H3C `NH2 H2N `NH2 CH3-0-CH2CH3 9b: Certain C-H functional groups can form weak hydrogen bonds. Why would such a functional group be more likely to be a H-bond donor when the C (carbon) is next to N (nitrogen)? (explain in 2-3 sentences) 9c: Explain why water forms nearly spherical droplets on the surface of a freshly waxed car. Define the effect from your knowledge in biochemistry (explain in 2-3 sentences)
Q9 9a: Rank the water solubility of the following compounds: al H3C H3C `NH2 H2N `NH2 CH3-0-CH2CH3 9b: Certain C-H functional groups can form weak hydrogen bonds. Why would such a functional group be more likely to be a H-bond donor when the C (carbon) is next to N (nitrogen)? (explain in 2-3 sentences) 9c: Explain why water forms nearly spherical droplets on the surface of a freshly waxed car. Define the effect from your knowledge in biochemistry (explain in 2-3 sentences)
Principles of Modern Chemistry
8th Edition
ISBN:9781305079113
Author:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Chapter14: Chemical Equilibrium
Section: Chapter Questions
Problem 93AP
Related questions
Question
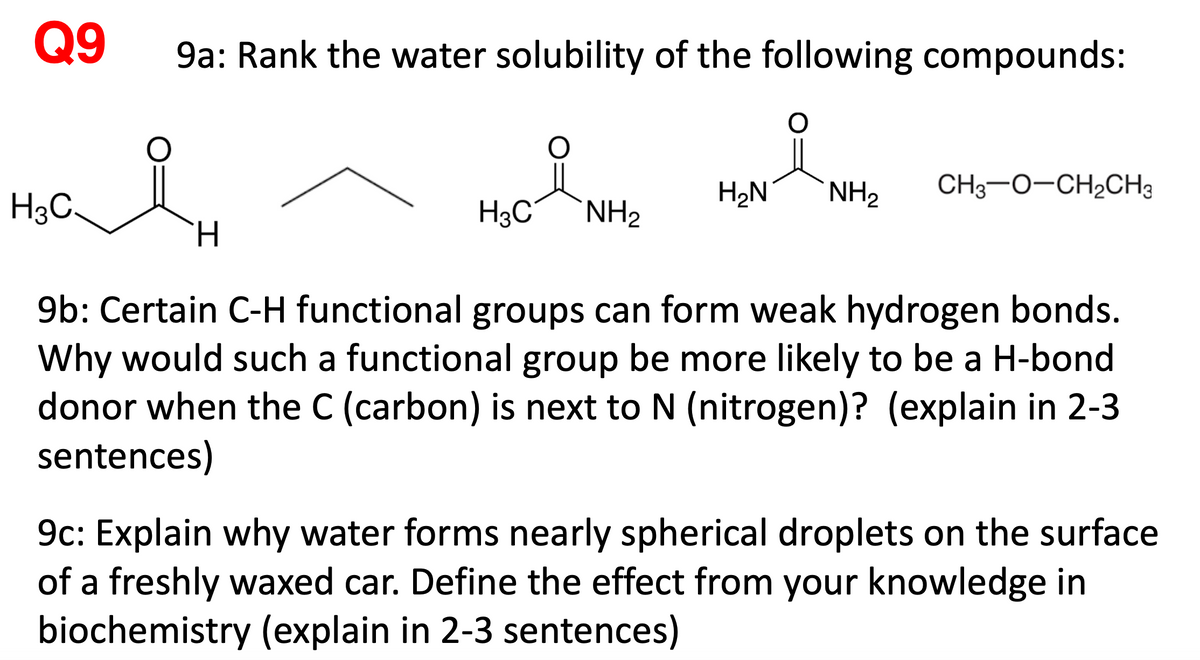
Transcribed Image Text:Q9
9a: Rank the water solubility of the following compounds:
H2N
NH2
CH3-0-CH2CH3
H3C.
H3C°
NH2
TH.
9b: Certain C-H functional groups can form weak hydrogen bonds.
Why would such a functional group be more likely to be a H-bond
donor when the C (carbon) is next to N (nitrogen)? (explain in 2-3
sentences)
9c: Explain why water forms nearly spherical droplets on the surface
of a freshly waxed car. Define the effect from your knowledge in
biochemistry (explain in 2-3 sentences)
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 3 images

Recommended textbooks for you

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Principles of Modern Chemistry
Chemistry
ISBN:
9781305079113
Author:
David W. Oxtoby, H. Pat Gillis, Laurie J. Butler
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning
