Qs3. Tell how you would use the proton NMR and IR spectra to distinguish between ethyl benzoate and 4- ethylbenzoic acid (drawing the structures will help you a lot). You can draw a schematic NMR spectrum for each compound, or simply list the peaks that shall be present (including integration and multiplicity).
Qs3. Tell how you would use the proton NMR and IR spectra to distinguish between ethyl benzoate and 4- ethylbenzoic acid (drawing the structures will help you a lot). You can draw a schematic NMR spectrum for each compound, or simply list the peaks that shall be present (including integration and multiplicity).
Chapter14: Chromatography
Section: Chapter Questions
Problem 6P
Related questions
Question
I really need help with #3 and #4
Transcribed Image Text:Qs3. Tell how you would use the proton NMR and IR spectra to distinguish between ethyl benzoate and 4-
ethylbenzoic acid (drawing the structures will help you a lot). You can draw a schematic NMR spectrum for
each compound, or simply list the peaks that shall be present (including integration and multiplicity).
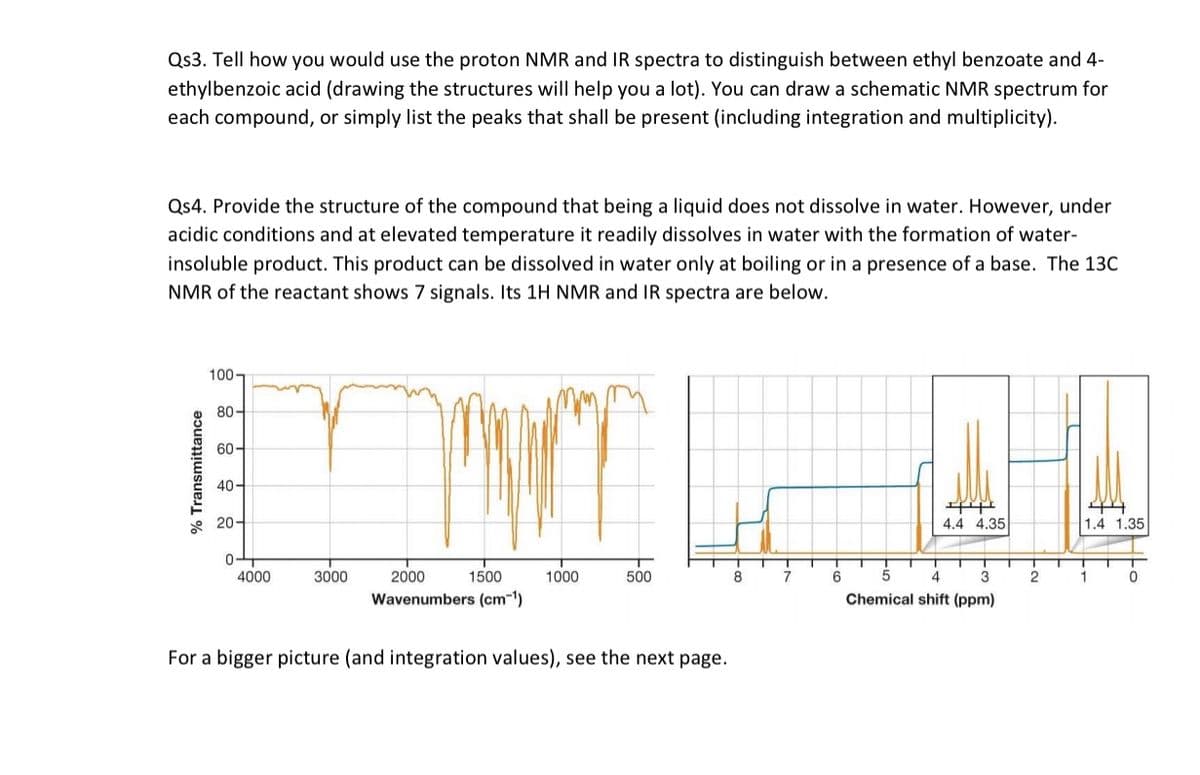
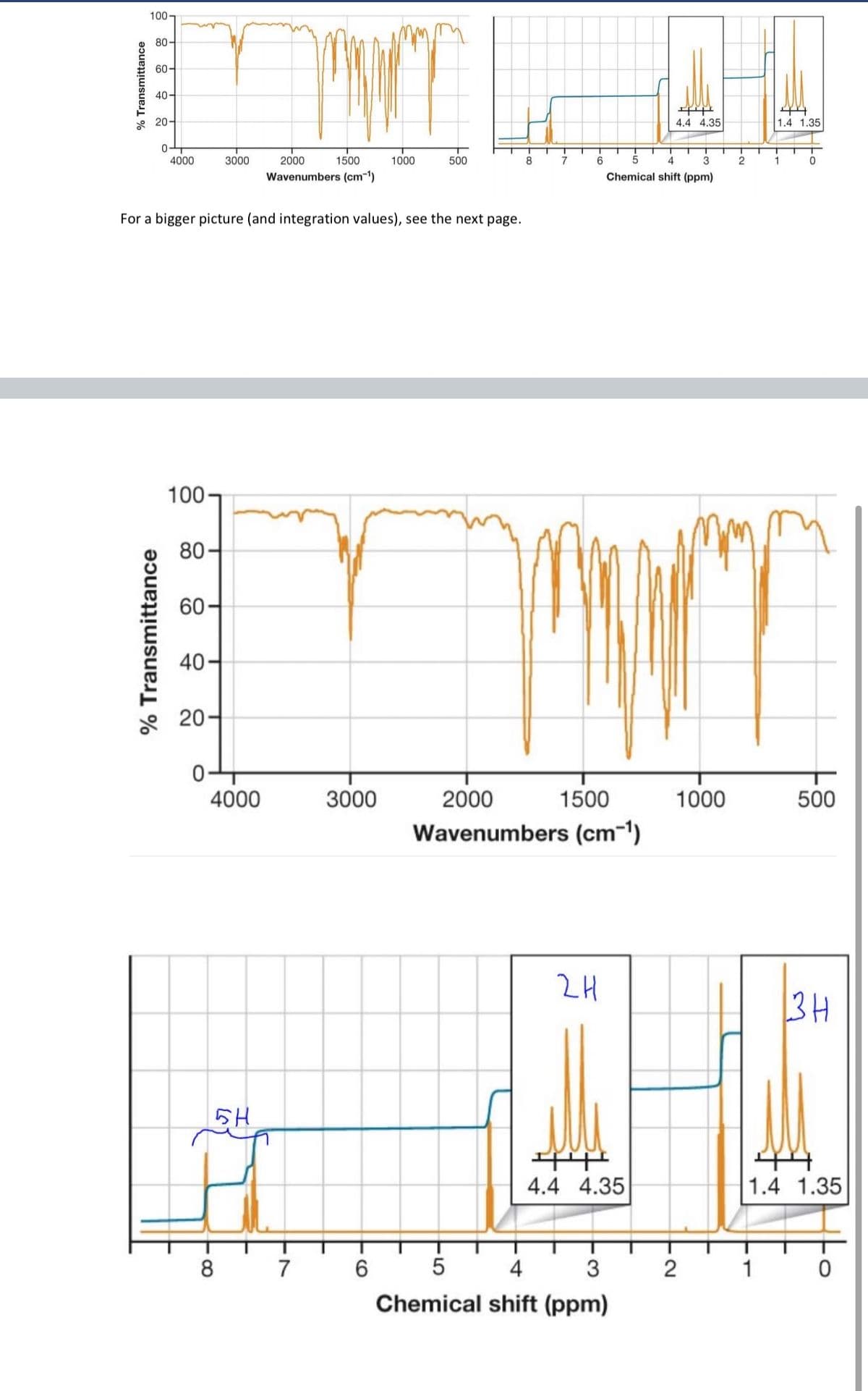
Qs4. Provide the structure of the compound that being a liquid does not dissolve in water. However, under
acidic conditions and at elevated temperature it readily dissolves in water with the formation of water-
insoluble product. This product can be dissolved in water only at boiling or in a presence of a base. The 13C
NMR of the reactant shows 7 signals. Its 1H NMR and IR spectra are below.
100-
80-
60-
40-
* 20-
4.4 4.35
1.4 1.35
4000
3000
2000
1500
1000
500
8.
7
6
3
2
Wavenumbers (cm-1)
Chemical shift (ppm)
For a bigger picture (and integration values), see the next page.
Transcribed Image Text:100-
80-
60-
40-
৪ 20-
4.4 4.35
1.4 1.35
0-
4000
3000
2000
1500
1000
500
8
7
6
4.
3
2
1
Wavenumbers (cm-1)
Chemical shift (ppm)
For a bigger picture (and integration values), see the next page.
100
80-
40-
20-
4000
3000
2000
1500
1000
500
Wavenumbers (cm-1)
2H
4.4 4.35
1.4 1.35
8 7
5
4
1
Chemical shift (ppm)
% Transmittance
% Transmittance
60
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 3 steps with 1 images

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning



Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning
