Question: 1. Determine the lambda max used to read the solutions. How was this obtained? 2. Determine the molar absorptivity coefficient of the metal complex at lambda max using the 5 standard solutions. (Suppose that path length = 1 cm)
Question: 1. Determine the lambda max used to read the solutions. How was this obtained? 2. Determine the molar absorptivity coefficient of the metal complex at lambda max using the 5 standard solutions. (Suppose that path length = 1 cm)
Chapter24: Introduction To Spectrochemical Methods
Section: Chapter Questions
Problem 24.16QAP
Related questions
Question
A metal complex, [Y(NH3)6]n+, gives a purple color. Suppose that Y is an unknown metal. A 0.0000258 M standard solution was scanned using UV-vis spectrometer, and the data collected is in Image 1.
Four more standard solutions were also scanned at a lambda max which is related to the puple color. The data for this is in Image 2.
Question:
1. Determine the lambda max used to read the solutions. How was this obtained?
2. Determine the molar absorptivity coefficient of the metal complex at lambda max using the 5 standard solutions. (Suppose that path length = 1 cm)

Transcribed Image Text:Concentration (x 10-5 M)
Absorbance
1.59
0.220
2.17
0.309
3.73
0.605
4.27
0.690
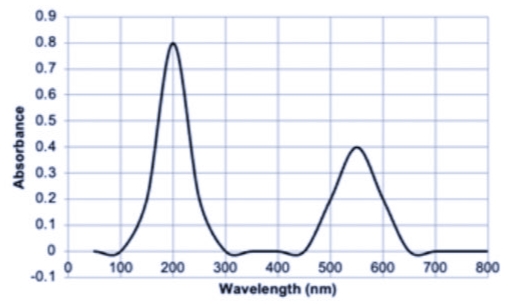
Transcribed Image Text:0.9
0.8
0.7
0.6
0.5
0.4
0.3
0.2
0.1
100
200
300
400
500
600
700
800
-0.1 0
Wavelength (nm)
Absorbance
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps

Knowledge Booster
Learn more about
Need a deep-dive on the concept behind this application? Look no further. Learn more about this topic, chemistry and related others by exploring similar questions and additional content below.Recommended textbooks for you


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning


Principles of Instrumental Analysis
Chemistry
ISBN:
9781305577213
Author:
Douglas A. Skoog, F. James Holler, Stanley R. Crouch
Publisher:
Cengage Learning