Question 13 of 31 How many atoms of carbon are produced by the complete reaction of 7.09 grams of magnesium turnings with excess dry ice (CO2), according to the balanced chemical reaction: 2 Mg(s) +CO2(s) 2 MgO(s) + C(s) 1 motMg 1 metC 6.022 x 102 atoms Mg = 3.56 x 1023 atoms C 24.31 2 motMg metC mol C 6.022 x 1023 atoms Mg = 3.56 x 1023 atoms C mol Mg 1 mol C ADD FACTOR DELETE ANSWER RESET 7.09 1.75 3.56 x 1023 3.50 40.30 1 8.78 x 1022 0.146 1.76 x 1023 44.01 0.292 12.01 2 24.31 6.022 x 1023 mol Mg g CO2 mol C atoms CO2 g Mg mol CO2 g Mgo atoms MgO mol Mgo atoms Mg atoms C
Question 13 of 31 How many atoms of carbon are produced by the complete reaction of 7.09 grams of magnesium turnings with excess dry ice (CO2), according to the balanced chemical reaction: 2 Mg(s) +CO2(s) 2 MgO(s) + C(s) 1 motMg 1 metC 6.022 x 102 atoms Mg = 3.56 x 1023 atoms C 24.31 2 motMg metC mol C 6.022 x 1023 atoms Mg = 3.56 x 1023 atoms C mol Mg 1 mol C ADD FACTOR DELETE ANSWER RESET 7.09 1.75 3.56 x 1023 3.50 40.30 1 8.78 x 1022 0.146 1.76 x 1023 44.01 0.292 12.01 2 24.31 6.022 x 1023 mol Mg g CO2 mol C atoms CO2 g Mg mol CO2 g Mgo atoms MgO mol Mgo atoms Mg atoms C
Chapter5: Gases
Section: Chapter Questions
Problem 161IP: In the presence of nitric acid, UO2+ undergoes a redox process. It is converted to UO22+ and nitric...
Related questions
Question
100%
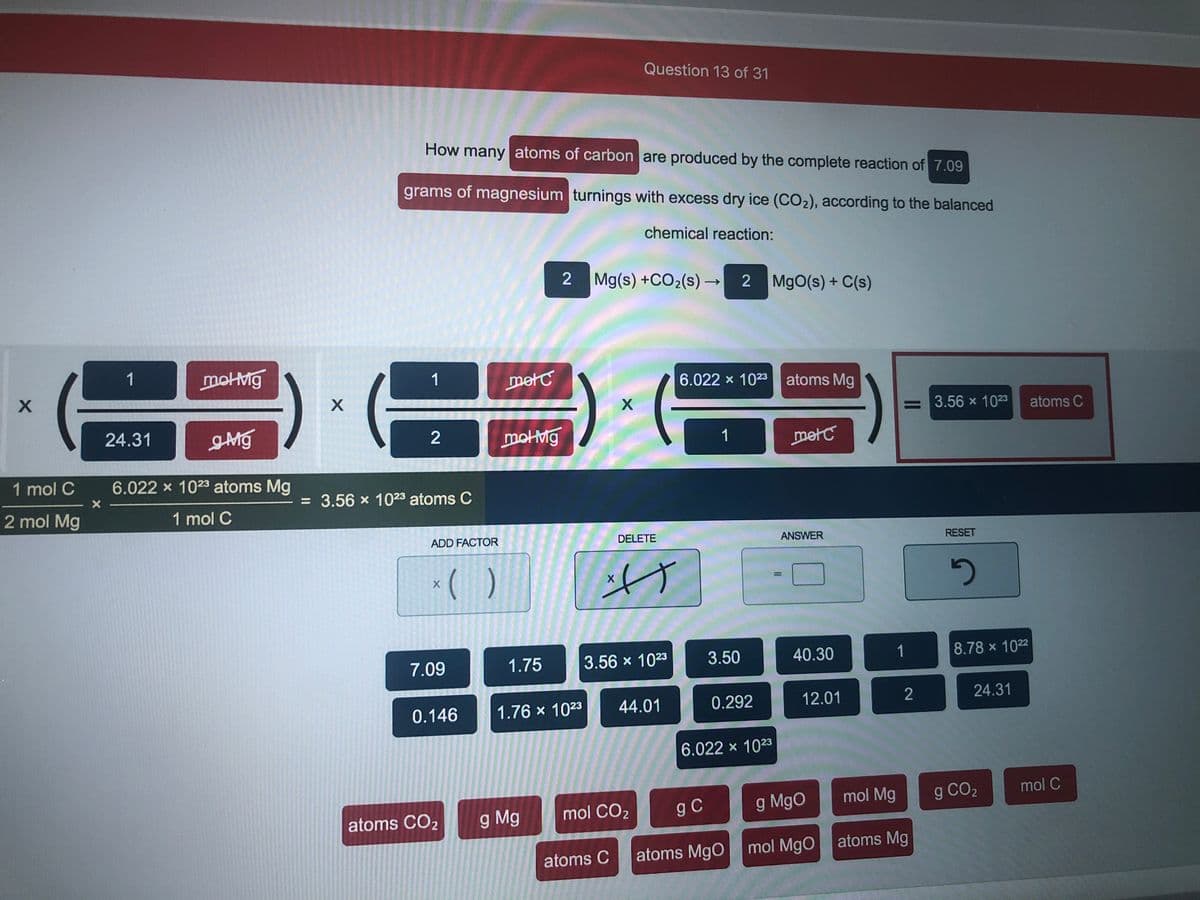
Transcribed Image Text:Question 13 of 31
How many atoms of carbon are produced by the complete reaction of 7.09
grams of magnesium turnings with excess dry ice (CO2), according to the balanced
chemical reaction:
2 Mg(s) +CO2(s) →
MgO(s) + C(s)
1
motMg
1
metC
6.022 x 1023 atoms Mg
= 3.56 x 1023
atoms C
24.31
2
motMg
1
metC
1 mol C
6.022 x 1023 atoms Mg
= 3.56 x 1023 atoms C
2 mol Mg
1 mol C
ADD FACTOR
DELETE
ANSWER
RESET
* ( )
7.09
1.75
3.56 x 1023
3.50
40.30
1
8.78 x 1022
0.146
1.76 x 1023
44.01
0.292
12.01
24.31
6.022 x 1023
mol CO2
g C
g MgO
mol Mg
g CO2
mol C
atoms CO2
g Mg
atoms MgO
mol MgO atoms Mg
atoms C
Expert Solution
This question has been solved!
Explore an expertly crafted, step-by-step solution for a thorough understanding of key concepts.
This is a popular solution!
Trending now
This is a popular solution!
Step by step
Solved in 2 steps with 2 images

Recommended textbooks for you


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning


Chemistry: An Atoms First Approach
Chemistry
ISBN:
9781305079243
Author:
Steven S. Zumdahl, Susan A. Zumdahl
Publisher:
Cengage Learning

Chemistry
Chemistry
ISBN:
9781305957404
Author:
Steven S. Zumdahl, Susan A. Zumdahl, Donald J. DeCoste
Publisher:
Cengage Learning

Living By Chemistry: First Edition Textbook
Chemistry
ISBN:
9781559539418
Author:
Angelica Stacy
Publisher:
MAC HIGHER

Chemistry: Principles and Reactions
Chemistry
ISBN:
9781305079373
Author:
William L. Masterton, Cecile N. Hurley
Publisher:
Cengage Learning

Chemistry by OpenStax (2015-05-04)
Chemistry
ISBN:
9781938168390
Author:
Klaus Theopold, Richard H Langley, Paul Flowers, William R. Robinson, Mark Blaser
Publisher:
OpenStax